W359904
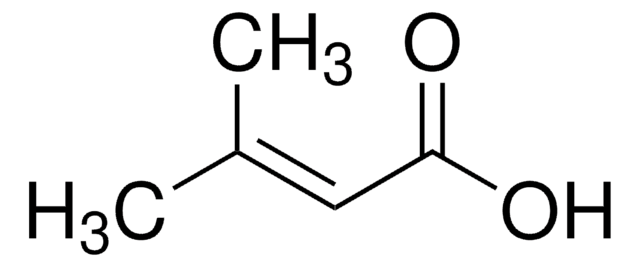
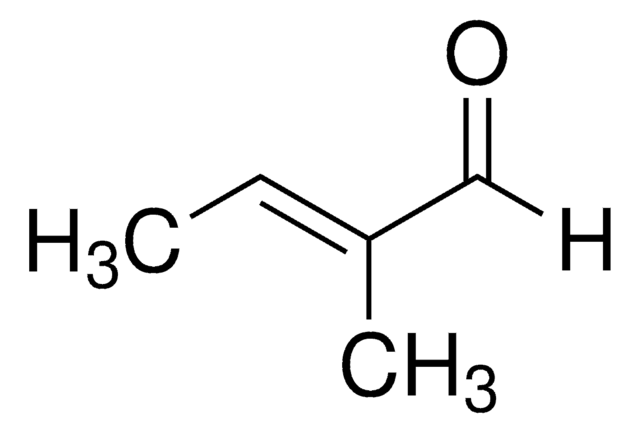
trans-2-Methyl-2-butenoic acid
≥99%, FG
Sinonimo/i:
Tiglic acid, trans-2,3-Dimethylacrylic acid, trans-2-Methyl-2-butenoic acid
About This Item
Prodotti consigliati
Origine biologica
synthetic
Grado
FG
Fragrance grade
Halal
Kosher
agenzia
follows IFRA guidelines
meets purity specifications of JECFA
Conformità normativa
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
Saggio
≥99%
P. ebollizione
95-96 °C/12 mmHg (lit.)
Punto di fusione
61-64 °C (lit.)
Densità
0.969 g/mL at 25 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Allergene in fragranze
no known allergens
Organolettico
brown; spicy
Stringa SMILE
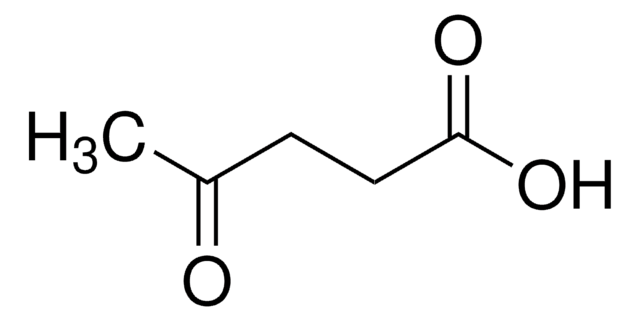
C\C=C(/C)C(O)=O
InChI
1S/C5H8O2/c1-3-4(2)5(6)7/h3H,1-2H3,(H,6,7)/b4-3+
UIERETOOQGIECD-ONEGZZNKSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- A high molar extinction coefficient bisterpyridyl homoleptic Ru(II) complex with trans-2-methyl-2-butenoic acid functionality: potential dye for dye-sensitized solar cells.: This study explores the synthesis and application of a Ru(II) complex incorporating trans-2-methyl-2-butenoic acid as a dye for dye-sensitized solar cells. The high molar extinction coefficient and favorable photophysical properties make this compound a promising candidate for enhancing solar cell efficiency (Adeloye et al., 2012).
- Synthesis, photophysical and electrochemical properties of a mixed bipyridyl-phenanthrolyl ligand Ru(II) heteroleptic complex having trans-2-methyl-2-butenoic acid functionalities.: This research focuses on the synthesis and characterization of a Ru(II) heteroleptic complex with trans-2-methyl-2-butenoic acid functionalities. The study highlights the compound′s photophysical and electrochemical properties, demonstrating its potential for use in optoelectronic devices and catalytic applications (Adeloye, 2011).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
203.0 °F
Punto d’infiammabilità (°C)
95 °C
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W359904-100G | |
| W359904-5KG-K | 4061834397779 |
| W359904-SAMPLE-K | 4061834406204 |
| W359904-100G-K | 4061835567232 |
| W359904-1KG | |
| W359904-1KG-K | 4061835567249 |
| W359904-5KG | |
| W359904-SAMPLE |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.