W318101
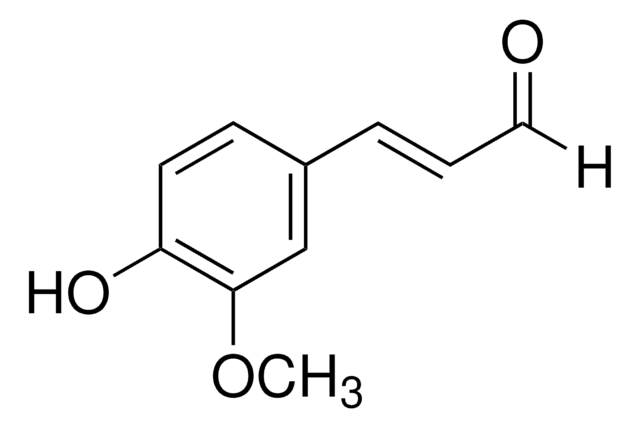
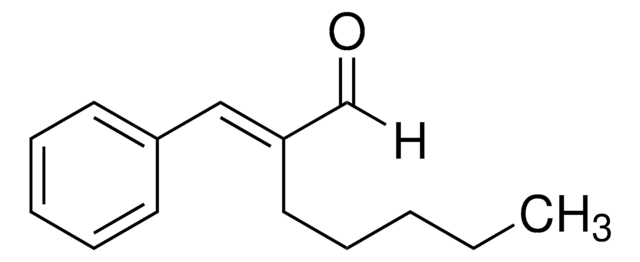
2-Methoxycinnamaldehyde
natural, 98%, FG
Sinonimo/i:
o-Methoxycinnamaldehyde
About This Item
Fragrance grade
Halal
Kosher
natural
Prodotti consigliati
Grado
FG
Fragrance grade
Halal
Kosher
natural
Livello qualitativo
agenzia
follows IFRA guidelines
Conformità normativa
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 172.515
Saggio
98%
P. ebollizione
160-161 °C/12 mmHg (lit.)
Punto di fusione
44.0-49.0 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Allergene in fragranze
no known allergens
Organolettico
cinnamon; woody; spicy; sweet
Stringa SMILE
[H]C(=O)C=Cc1ccccc1OC
InChI
1S/C10H10O2/c1-12-10-7-3-2-5-9(10)6-4-8-11/h2-8H,1H3/b6-4+
KKVZAVRSVHUSPL-GQCTYLIASA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- Network pharmacology combined with molecular docking and experimental validation to explore the potential mechanism of Cinnamomi ramulus against ankylosing spondylitis.: This study investigates the anti-inflammatory potential of 2-Methoxycinnamaldehyde in Cinnamomi ramulus. Its application extends to novel therapeutic approaches for treating ankylosing spondylitis, demonstrating significant implications for medicinal chemistry and pharmacology (Wei et al., 2023).
- Ramulus Cinnamomi essential oil exerts an anti-inflammatory effect on RAW264.7 cells through N-acylethanolamine acid amidase inhibition.: The study elaborates on the anti-inflammatory activities of 2-Methoxycinnamaldehyde, offering a biochemical pathway that could be exploited in anti-inflammatory drug design (Jia et al., 2023).
- Metabolomics-Driven Exploration of the Antibacterial Activity and Mechanism of 2-Methoxycinnamaldehyde.: This article offers insights into the antibacterial properties of 2-Methoxycinnamaldehyde, using metabolomics to explore its mechanism of action, significant for developments in antimicrobial treatments (Qian et al., 2022).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
235.4 °F
Punto d’infiammabilità (°C)
113 °C
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.