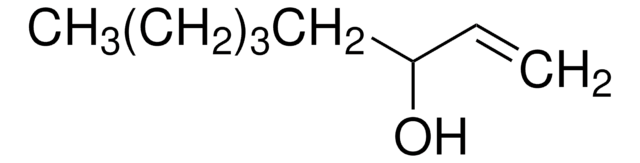W280523
1-Octen-3-ol
natural, ≥95%, FG
Sinonimo/i:
Pentyl vinyl carbinol
About This Item
Prodotti consigliati
Grado
FG
Fragrance grade
Halal
Kosher
natural
agenzia
follows IFRA guidelines
Conformità normativa
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 172.515
Saggio
≥95%
Caratteristiche più verdi
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Indice di rifrazione
n20/D 1.437 (lit.)
P. ebollizione
84-85 °C/25 mmHg (lit.)
Densità
0.837 g/mL at 20 °C
0.83 g/mL at 25 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Allergene in fragranze
no known allergens
Categoria alternativa più verde
Organolettico
mushroom; musty; earthy
Stringa SMILE
CCCCCC(O)C=C
InChI
1S/C8H16O/c1-3-5-6-7-8(9)4-2/h4,8-9H,2-3,5-7H2,1H3
VSMOENVRRABVKN-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Fluctuation of flavor quality in roasted duck: The consequences of raw duck preform′s repetitive freeze-thawing.: This study explores how repetitive freeze-thaw cycles of raw duck preforms influence the flavor quality of roasted duck, examining specific volatile compounds including 1-Octen-3-ol and its impact on sensory attributes (Gao et al., 2024).
- A new HS-SPME-GC-MS analytical method to identify and quantify compounds responsible for changes in the volatile profile in five types of meat products during aerobic storage at 4 °C.: This research introduces a novel analytical approach using HS-SPME-GC-MS to track changes in volatile profiles, including the role of 1-Octen-3-ol, across different meat products under specific storage conditions (Acquaticci et al., 2024).
- Comprehensive investigation on the dynamic changes of volatile metabolites in fresh scent green tea during processing by GC-E-Nose, GC-MS, and GC × GC-TOFMS.: This detailed study assesses how volatile metabolites like 1-Octen-3-ol evolve during the processing of fresh scent green tea, utilizing advanced gas chromatography techniques (Wang et al., 2024).
- Insights into "wheat aroma": Analysis of volatile components in wheat grains cultivated in saline-alkali soil.: This article examines the ′wheat aroma′ by analyzing volatile components, including 1-Octen-3-ol, in wheat grains grown in challenging saline-alkali soils, highlighting the adaptative traits of these crops (Sun et al., 2024).
- Demonstrating the Applicability of Proton Transfer Reaction Mass Spectrometry to Quantify Volatiles Emitted by the Mycoparasitic Fungus Trichoderma atroviride in Real Time: Monitoring of Trichoderma-Based Biopesticides.: This study demonstrates the use of proton transfer reaction mass spectrometry to quantify volatile organic compounds, including 1-Octen-3-ol, emitted in real-time by the fungus Trichoderma atroviride, used in biopesticide applications (Lochmann et al., 2024).
Altre note
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Skin Irrit. 2 - Skin Sens. 1
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
154.4 °F - closed cup
Punto d’infiammabilità (°C)
68 °C - closed cup
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W280523-SAMPLE-K | 4061834355731 |
| W280523-100G-K | 4061834404910 |
| W280523-1KG-K | 4061833904466 |
| W280523-5KG-K |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.