C95501
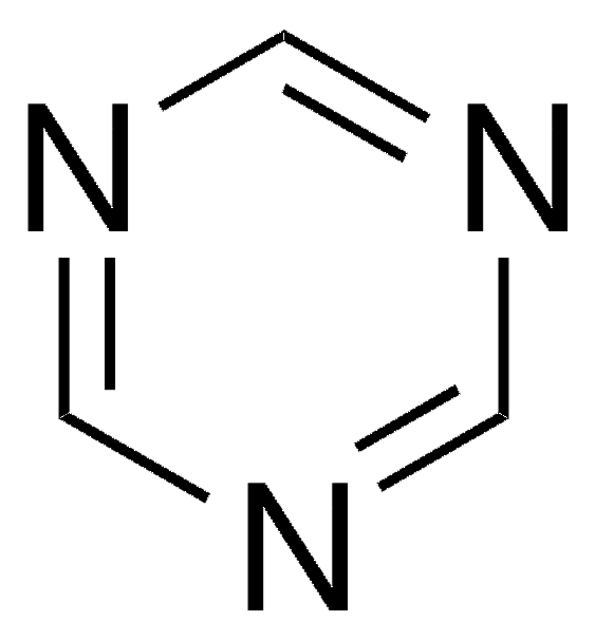
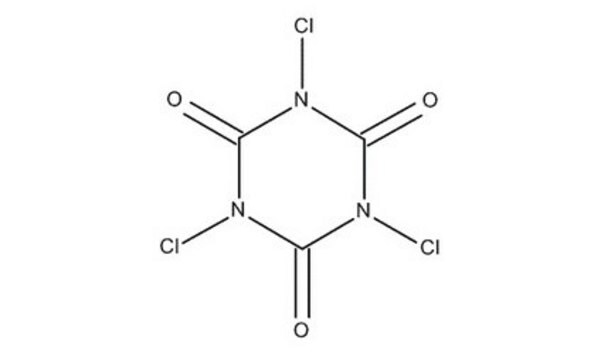
Cyanuric chloride
99%
Sinonimo/i:
2,4,6-Trichloro-1,3,5-triazine
About This Item
Prodotti consigliati
Densità del vapore
6.36 (vs air)
Livello qualitativo
Tensione di vapore
0.8 mmHg ( 62.2 °C)
Saggio
99%
Forma fisica
powder
P. eboll.
190 °C (lit.)
Punto di fusione
145-147 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
Clc1nc(Cl)nc(Cl)n1
InChI
1S/C3Cl3N3/c4-1-7-2(5)9-3(6)8-1
MGNCLNQXLYJVJD-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- In the preparation of acyl azides from carboxylic acids and sodium azide.
- For the conversion of carboxylic acids, N-Boc, N-Cbz, and N-Fmoc amino acids into corresponding alcohols.
- For the conversion of alcohols into the corresponding carbonyl compounds by alternative Swern oxidation reaction.
It can also be employed as a catalyst in the Beckmann rearrangement of ketoximes into amides in the presence of ZnCl2.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1A - STOT SE 3
Organi bersaglio
Respiratory system
Rischi supp
Codice della classe di stoccaggio
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
>392.0 °F - closed cup
Punto d’infiammabilità (°C)
> 200 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Collagen molecules play a critical role in tissue architecture and strength, and in cell-matrix interactions as insoluble ligands to regulate the diverse phenotypic activities of cells.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.