C58002
6-Chloronicotinamide
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
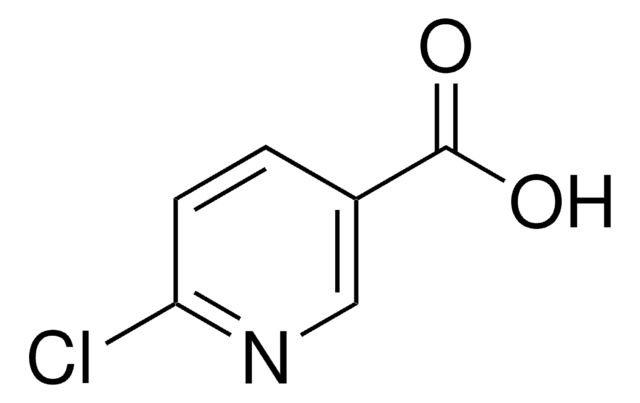
Formula empirica (notazione di Hill):
C6H5ClN2O
Numero CAS:
Peso molecolare:
156.57
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Forma fisica
powder
Punto di fusione
210-212 °C (lit.)
Stringa SMILE
NC(=O)c1ccc(Cl)nc1
InChI
1S/C6H5ClN2O/c7-5-2-1-4(3-9-5)6(8)10/h1-3H,(H2,8,10)
ZIJAZUBWHAZHPL-UHFFFAOYSA-N
Categorie correlate
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Heather S Loring et al.
Biochemistry, 57(38), 5524-5532 (2018-08-28)
Nicotinamide N-methyltransferase (NNMT) catalyzes the transfer of a methyl group from S-adenosylmethionine (SAM) to nicotinamide, pyridine, and other structural analogues. Aberrantly increased NNMT activity results in the depletion of SAM, nicotinamide (NAM), and nicotinamide adenine dinucleotide (NAD+); NAM is required
Angela Fabiano et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 130, 281-289 (2018-07-15)
Nanoparticles (NP) only different in mucoadhesivity are compared for impact on drug oral bioavailability. Two polymeric NP types based on quaternary ammonium-chitosan (NP QA-Ch) and S-protected thiolated derivative thereof (NP QA-Ch-S-pro), respectively, containing the macromolecular drug model, FD4, were prepared
Harshini Neelakantan et al.
Biochemical pharmacology, 147, 141-152 (2017-11-21)
There is a critical need for new mechanism-of-action drugs that reduce the burden of obesity and associated chronic metabolic comorbidities. A potentially novel target to treat obesity and type 2 diabetes is nicotinamide-N-methyltransferase (NNMT), a cytosolic enzyme with newly identified
Entirely S-protected chitosan: A promising mucoadhesive excipient for metronidazole vaginal tablets.
Noemi Lupo et al.
Acta biomaterialia, 64, 106-115 (2017-10-17)
Synthesis and evaluation of an entirely S-protected chitosan as mucoadhesive excipient for vaginal drug delivery. N-acetyl-cysteine was linked to 6-mercaptonicotinamide via disulphide exchange reaction. The obtained ligand, NAC-6-MNA, was subsequently attached to chitosan by carbodiimide mediated amide bond formation in
Flavia Laffleur et al.
Journal of pharmaceutical sciences, 103(8), 2414-2423 (2014-07-06)
This study was aimed to investigate chemical preactivated thiomers for their potential use in mucosal drug delivery. Thiomers--thiolated polymers--are mucoadhesive polymers with sulfhydryl group-bearing side chains. Thiomers are synthesized by covalent attachment of low molecular mass compounds bearing sulfhydryl group
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.