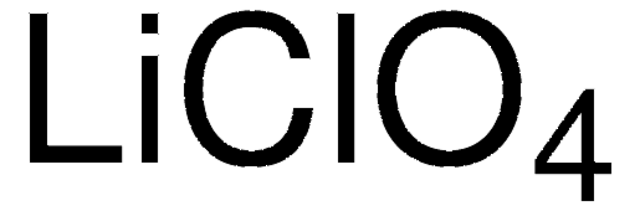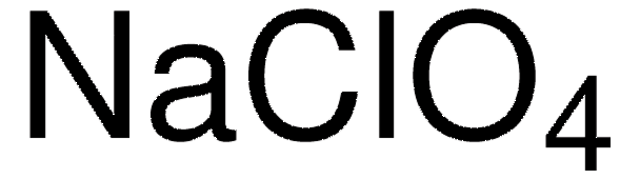931969
Lithium perchlorate
anhydrous, ≥99.9% trace metals basis
Sinonimo/i:
Perchloric acid lithium salt
About This Item
Prodotti consigliati
Grado
anhydrous
battery grade
Livello qualitativo
Saggio
≥99.9% trace metals basis
Forma fisica
powder
Caratteristiche più verdi
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Impurezze
≤1000 ppm (trace metals analysis)
pH
6.0-7.5 (25 °C, 5%, aq.sol.)
Punto di fusione
236 °C (lit.)
Solubilità
H2O: 59.8 g/dL at 25 °C
Anioni in tracce
chloride (Cl-): ≤30 ppm
sulfate (SO42-): ≤10 ppm
Cationi in tracce
Fe: ≤5 ppm
heavy metals: ≤10 ppm
applicazioni
battery manufacturing
Categoria alternativa più verde
Stringa SMILE
[Li+].[O-]Cl(=O)(=O)=O
InChI
1S/ClHO4.Li/c2-1(3,4)5;/h(H,2,3,4,5);/q;+1/p-1
MHCFAGZWMAWTNR-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Industrially, lithium perchlorate is manufactured in several ways. Most commonly, it is prepared from sodium perchlorate through a metathesis reaction with lithium chloride or lithium carbonate. Lithium perchlorate can also be prepared by direct electrochemical oxidation of lithium chloride or by reacting lithium carbonate with perchloric acid. The hydrate can be dried either by highly controlled heating or by displacing water with volatile amines, which are removed by drying under vacuum.
Applicazioni
Researchers also use lithium perchlorate as an electrolytic salt in aqueous media when testing electrocatalysts. For example, recent experiments improving the electrochemical reduction of nitrogen over TiO2 nanoparticles or gold nanoparticles use aqueous lithium perchlorate as the electrolyte.
Confezionamento
500g in poly bottle
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1 - Ox. Sol. 2 - Skin Corr. 1A - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
5.1A - Strongly oxidizing hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.