907391
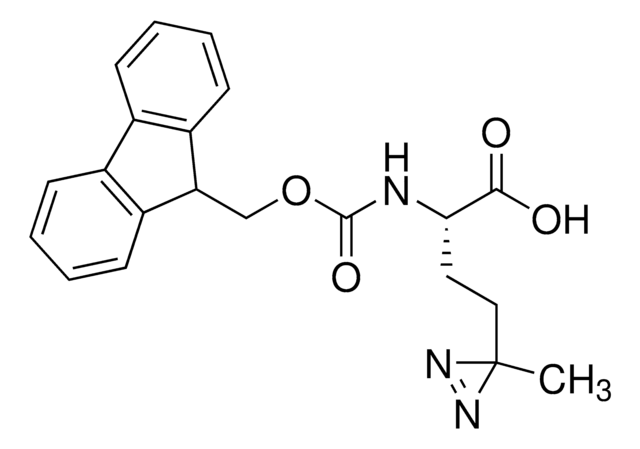
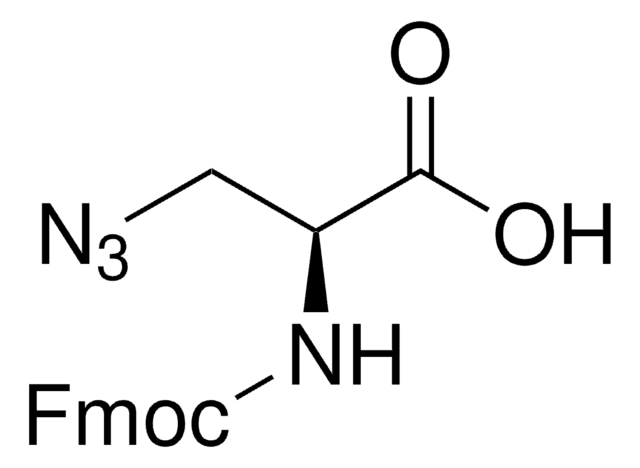
Fmoc-L-photo-leucine
≥98%
Sinonimo/i:
(S)-2-(((9H-Fluoren-9-yl)methoxy)carbonylamino)-3-(3-methyl-3H-diazirin-3-yl)propanoic acid, (S)-2-(Fmoc-amino)-3-(3H-diazirin-3-yl)butanoic acid, Photo-Leu, Photo-crosslinking amino acid, Photoprobe building block
About This Item
Prodotti consigliati
Saggio
≥98%
Forma fisica
powder
Impiego in reazioni chimiche
reaction type: Fmoc solid-phase peptide synthesis
applicazioni
peptide synthesis
Gruppo funzionale
Fmoc
Temperatura di conservazione
2-8°C
Applicazioni
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Altre note
Developing diazirine-based chemical probes to identify histone modification ′readers′ and ′erasers′
Protein-Polymer Conjugation via Ligand Affinity and Photoactivation of Glutathione S-Transferase
Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis
Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs)
Prodotti correlati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.