807664
CBTF
Sinonimo/i:
APN-TFS ester, Sodium 4-((4-(cyanoethynyl)benzoyl)oxy)-2,3,5,6-tetrafluorobenzenesulfonate
About This Item
Prodotti consigliati
Stato
powder
Livello qualitativo
Impiego in reazioni chimiche
reagent type: cross-linking reagent
Gruppo funzionale
ester
Temperatura di conservazione
−20°C
Stringa SMILE
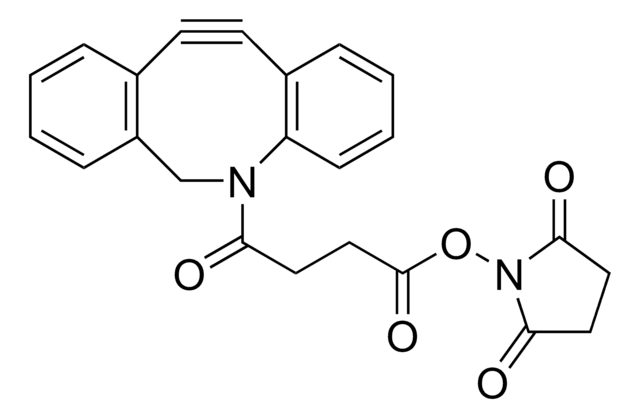
O=C(OC1=C(F)C(F)=C(S(=O)(O[Na])=O)C(F)=C1F)C2=CC=C(C#CC#N)C=C2
InChI
1S/C16H5F4NO5S.Na/c17-10-12(19)15(27(23,24)25)13(20)11(18)14(10)26-16(22)9-5-3-8(4-6-9)2-1-7-21;/h3-6H,(H,23,24,25);/q;+1/p-1
YFJYSJRZDOWXDH-UHFFFAOYSA-M
Descrizione generale
Applicazioni
Altre note
- Dissolve the protein in an appropriate buffer* with pH 7.5-9.0 (e.g. PBS) at 1-10mg/mL concentration.
- Apply the appropriate amount of the stock solution of the reagent (1-5 molar eq. per lysine residue).
- Incubate at room temperature for 2 hours.
- If necessary, purify the protein-APN conjugate using size exclusion chromatography or ultrafiltration.
- The conjugate can be readily coupled with thiol-containing substrates by incubating the components in aqueous buffer (pH 6.5-9.0) at ambient temperature for 2 hours.
Standard protein labeling procedure (cysteine labeling):
Dissolve the protein in the appropriate buffer* with pH 6.5-9.0 (e.g. PBS) at 1-10mg/mL concentration.
Apply the appropriate amount of the stock solution of the APN-labeled molecule (1-5 molar eq. per free cysteine residue).
Incubate at room temperature for 2 hours.
If necessary, purify the protein conjugate using size exclusion chromatography or ultrafiltration.
*Note: avoid amine- and thiol-containing buffers.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.![LC-SPDP (succinimidyl 6-[3(2-pyridyldithio)propionamido]hexanoate)](/deepweb/assets/sigmaaldrich/product/structures/300/586/d95fd80c-e201-4b0b-8aee-31e109c2ff41/640/d95fd80c-e201-4b0b-8aee-31e109c2ff41.png)






![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)