791806
Yu-Wasa Auxiliary
97%
Sinonimo/i:
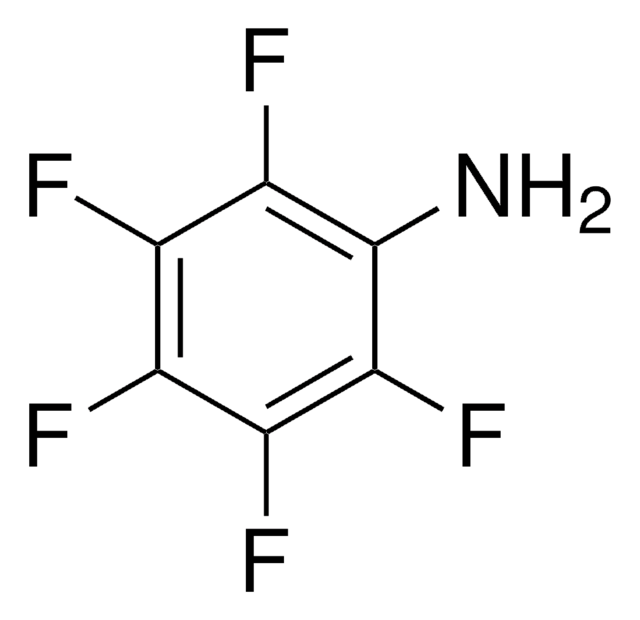
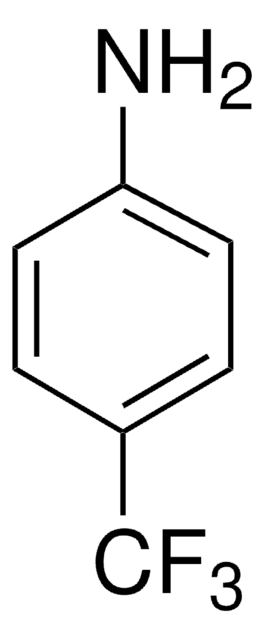
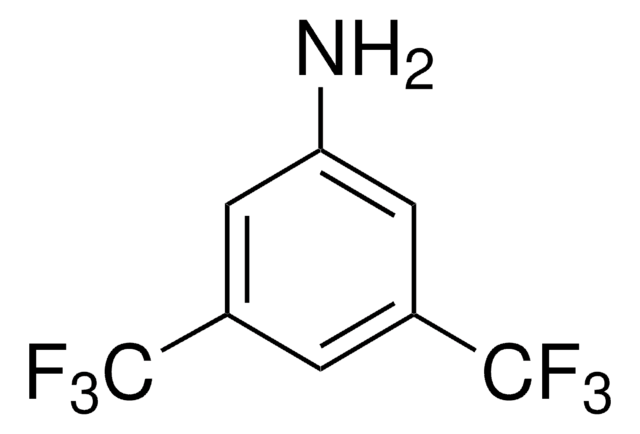
2,3,5,6-Tetrafluoro-4-(trifluoromethyl)aniline, 2,3,5,6,α,α,α-Heptafluoro-p-toluidine
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Forma fisica
liquid
Impiego in reazioni chimiche
reaction type: C-C Bond Formation
reagent type: catalyst
reaction type: C-H Activation
Indice di rifrazione
n20/D 1.431 (lit.)
n20/D 1.432
P. eboll.
186 °C (lit.)
Densità
1.662 g/mL at 25 °C
1.687 g/mL at 25 °C (lit.)
Gruppo funzionale
fluoro
Stringa SMILE
Nc1c(F)c(F)c(c(F)c1F)C(F)(F)F
InChI
1S/C7H2F7N/c8-2-1(7(12,13)14)3(9)5(11)6(15)4(2)10/h15H2
FJOACTZFMHZHSC-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Altre note
Used in the Preparation of
- Lactams via palladium-catalyzed olefination of arylamides with benzylacrylate, followed by 1,4-conjugate addition
- N-(fluorinated aryl)benzamides as substrates for regioselective C-H amination reactions with O-benzoylhydroxylamines
- Substituted succinimides via palladium-catalyzed carbonylation of N-aryl amides
- N-aryl cyclopropanecarboxamide substrates and various amino acid ligands for palladium-catalyzed C-H activation of cyclopropanes
Prodotti correlati
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.