775525
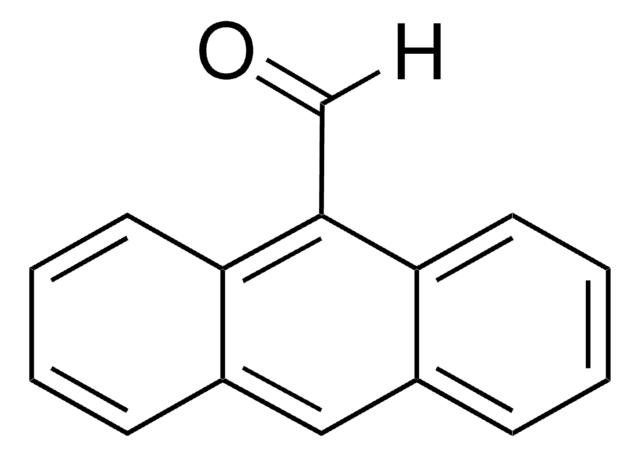
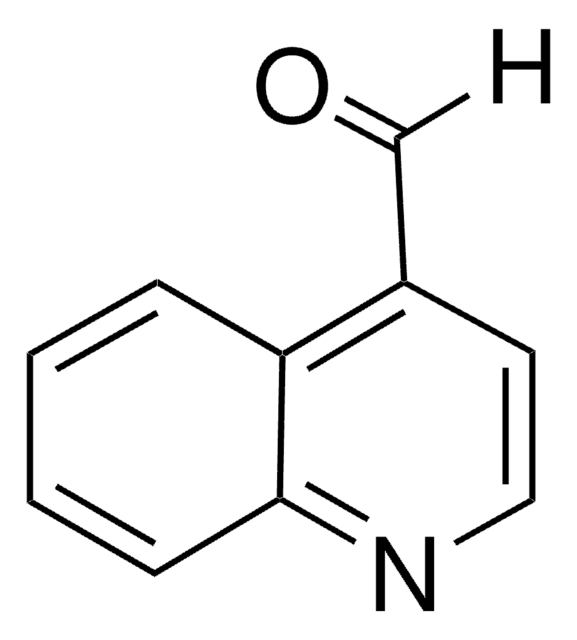
Acridine-9-carboxaldehyde
97%
Sinonimo/i:
Acridine-9-carbaldehyde
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C14H9NO
Numero CAS:
Peso molecolare:
207.23
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Forma fisica
solid
Punto di fusione
144-149 °C
Gruppo funzionale
aldehyde
Temperatura di conservazione
2-8°C
Stringa SMILE
O=Cc1c2ccccc2nc3ccccc13
InChI
1S/C14H9NO/c16-9-12-10-5-1-3-7-13(10)15-14-8-4-2-6-11(12)14/h1-9H
ISOCABSXIKQOOV-UHFFFAOYSA-N
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
S M Furst et al.
International journal of immunopharmacology, 17(5), 445-452 (1995-05-01)
Carbamazepine, a widely used anticonvulsant, is associated with a wide range of adverse reactions including agranulocytosis, aplastic anemia and drug-induced lupus. It has also been reported to alter immune function in a variety of ways. We had previously demonstrated that
Olivier Mathieu et al.
Xenobiotica; the fate of foreign compounds in biological systems, 41(2), 91-100 (2010-11-23)
Carbamazepine (CBZ) is a useful anticonvulsive drug associated with rare severe adverse drug reactions. The physio-pathological mechanisms of these reactions are unknown although evidence of immunological activation has been reported. The ability of 9-acridinecarboxaldehyde, a CBZ metabolite, to interact with
Olivier Mathieu et al.
Pharmacological reports : PR, 63(1), 86-94 (2011-03-29)
Carbamazepine is a widely used anticonvulsive agent. Its metabolic pathway leads not only to the major active metabolite, carbamazepine-10,11-epoxide, but also to minor terminal metabolites such as iminostilbene and acridine. Carbamazepine is usually well-tolerated, but it may lead to rare
Wolf-Ulrich Palm
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 17(7), 964-974 (2018-06-20)
Dark and photolysis reactions in solution were investigated for 9-acridinecarboxaldehyde (ACL). ACL reacts in the dark at T = 20 °C and pH = 7.0 in an air saturated solution to the main product 9-acridinecarboxylic acid (ACA) and to the
Mei Lu et al.
Journal of the American Society for Mass Spectrometry, 26(10), 1676-1685 (2015-08-06)
Electrochemistry (EC) combined with mass spectrometry (MS) is a powerful tool for elucidation of electrochemical reaction mechanisms. However, direct online analysis of electrochemical reaction in aqueous phase was rarely explored. This paper presents the online investigation of several electrochemical reactions
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.