76070
Palladium(II) nitrate dihydrate
~40% Pd basis
Sinonimo/i:
Palladium dinitrate hydrate
About This Item
Prodotti consigliati
Grado
for analytical purposes
Stato
powder or crystals
Impiego in reazioni chimiche
reagent type: catalyst
core: palladium
Concentrazione
~40% Pd
Stringa SMILE
O.O.[Pd++].[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/2NO3.2H2O.Pd/c2*2-1(3)4;;;/h;;2*1H2;/q2*-1;;;+2
JUBNUQXDQDMSKL-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
It is also employed in the fabrication of low-cost solid-state hydrogen storage devices made of three-dimensional (3D) reduced graphene oxide (rGO) and expanded graphite (EG)nanocomposite (NC) decorated with Pd nanoparticles (NPs).
Applicazioni
- Doping activated carbon for catalysis
- Catalytic use of hydroxyapatite (HAP) supported Pd nanoclusters in the hydrolysis of ammonia-borane.
It′s used as catalyst for:
- Selective hydrogenation of 1-heptyne and napthalene
- In highly porous coordination polymer MIL-101 support.
It may also be used as a reactant for:
- Preparation of platinum-palladium/carbon alloy nanocatalysts for methanol-tolerant oxygen reduction reaction in fuel cells
- Synthesis of Cu-Pd alloy thin films on Ti substrates by co-electrodeposition of Pd and Cu from nitrate-base electrolytic baths
- Preparation of palladium catalyst supported on vertically aligned multi-walled carbon nanotubes for microwave-assisted Heck reactions of p-iodonitrobenzene with styrene and Et acrylate
- Preparation of di-phenyl sulfide-modified Pd/TiO2 catalysts for acetylene hydrogenation
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Met. Corr. 1 - Ox. Sol. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
5.1A - Strongly oxidizing hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.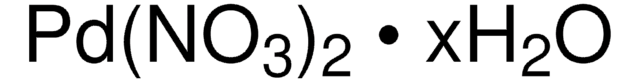




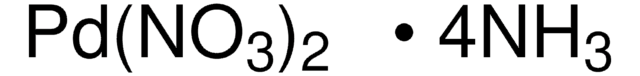






![[Pd(acac)2] Umicore, 99%](/deepweb/assets/sigmaaldrich/product/structures/145/685/c3d0f078-c0c6-4ce6-9c4a-b6a1b973b3a9/640/c3d0f078-c0c6-4ce6-9c4a-b6a1b973b3a9.png)