735094
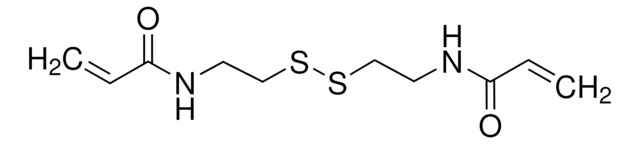
Bis(2-methacryloyl)oxyethyl disulfide
contains ≤6000 ppm hydroquinone as stabilizer
Sinonimo/i:
DSDMA, Disulfide-based dimethacrylate
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C12H18O4S2
Numero CAS:
Peso molecolare:
290.40
Numero MDL:
Codice UNSPSC:
12162002
ID PubChem:
NACRES:
NA.23
Prodotti consigliati
Forma fisica
liquid
contiene
≤6000 ppm hydroquinone as stabilizer
Indice di rifrazione
n20/D 1.517
Densità
1.141 g/mL at 25 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(=C)C(=O)OCCSSCCOC(=O)C(C)=C
InChI
1S/C12H18O4S2/c1-9(2)11(13)15-5-7-17-18-8-6-16-12(14)10(3)4/h1,3,5-8H2,2,4H3
CGDNFXSLPGLMHK-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Bis(2-methacryloyl)oxyethyl disulfide (DSDMA) belongs to the class of monomers known as disulfide-based dimethacrylates. It is widely employed as a crosslinker in the synthesis of various polymers with specific properties such as redox sensitivity and self-healing properties. DSDMA contains a disulfide bond, which can be cleaved under specific conditions, making it useful for drug delivery systems. It also undergoes thiol-disulfide exchange reactions, allowing it to react with thiols in polymers and form covalent crosslinks. This property enables the formation of networks and gels in polymer systems. Additionally, DSDMA is employed in the development of biomedical materials, such as tissue engineering scaffolds. Its ability to form stable crosslinks in biological environments makes it suitable for these applications.
Applicazioni
Bis(2-methacryloyl)oxyethyl disulfide (DSDMA) can be used in the following applications:
- Used as a crosslinker in the synthesis of reduction-responsive molecularly imprinted polymer (MIPs) nanogels for drug delivery applications. This reduction-responsive property allows for control over drug delivery and modulation of the release properties of the MIPs.
- Used as a crosslinker in the synthesis of self-healing polymer nanocomposites via dynamic disulfide exchange reaction and crosslinking properties. These self-healing polymer nanocomposites can be used in coatings, electronics, and packaging applications.
- Used as a redox-responsive cross-linker in the synthesis of zwitterionic hydrogels for effective drug delivery. DSDMA provides structural stability, redox-responsiveness, and self-healing properties, which are essential for effective drug delivery.
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 2
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Wenwen Li; Krzysztof Matyjaszewski; Krystyna Albrecht; Martin Moller
Macromolecules, 42, 8228-8228 (2009)
Kunihiko Kobayashi et al.
ACS applied materials & interfaces, 11(1), 151-159 (2018-12-12)
Soft-robotic devices such as polymeric microgrippers offer the possibility for pick and place of fragile biological cargo in hard-to-reach conduits with potential applications in drug delivery, minimally invasive surgery, and biomedical engineering. Previously, millimeter-sized self-folding thermomagnetically responsive soft grippers have
David S Spencer et al.
Journal of polymer science. Part A, Polymer chemistry, 56(14), 1536-1544 (2019-03-25)
Crosslinked cationic nanoscale networks with hydrophobic cores are an environmentally robust alternative to self-assembled polymeric drug delivery carriers with respect to therapeutic encapsulation and stability to dilution. However, the ability to tune the degree of PEG incorporated into nanogels during
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.