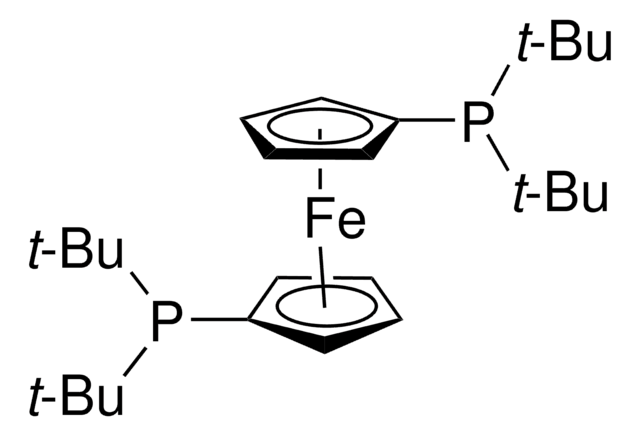
701602
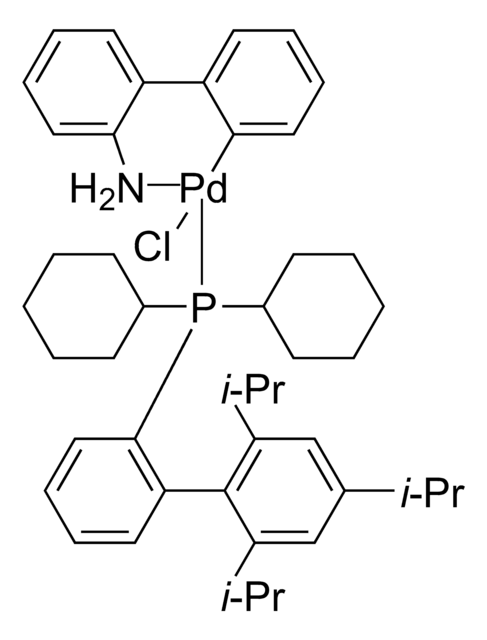
1,1′-Bis-(di-tert.-butylphosphino-)ferrocen-palladiumdichlorid
98%
Sinonimo/i:
PdCl2(dtbpf)
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
powder
Impiego in reazioni chimiche
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
Caratteristiche più verdi
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Punto di fusione
203-208 °C
Categoria alternativa più verde
, Aligned
Temperatura di conservazione
−20°C
Stringa SMILE
[Fe].Cl[Pd]Cl.CC(C)(C)P([C]1[CH][CH][CH][CH]1)C(C)(C)C.CC(C)(C)P([C]2[CH][CH][CH][CH]2)C(C)(C)C
InChI
1S/2C13H22P.2ClH.Fe.Pd/c2*1-12(2,3)14(13(4,5)6)11-9-7-8-10-11;;;;/h2*7-10H,1-6H3;2*1H;;/q;;;;;+2/p-2
JQZFOBWXNREQLO-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
PdCl2(dtbpf) is an air-stable cross-coupling catalyst used in the Suzuki coupling of various aryl chlorides.
Applicazioni
It is also employed as catalyst for greener Suzuki cross-coupling in TPGS-750-M.
On the Way Towards Greener Transition-Metal-Catalyzed Processes as Quantified by E Factors
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 701602-250MG | 4061833565100 |
| 701602-1G | 4061826700914 |
| 701602-50G | 4061833411605 |
| 701602-5G | 4061833330906 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II) complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)