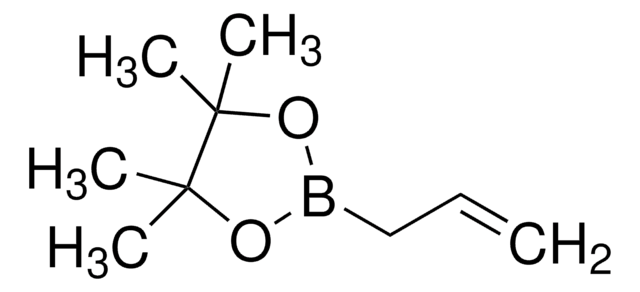
663212
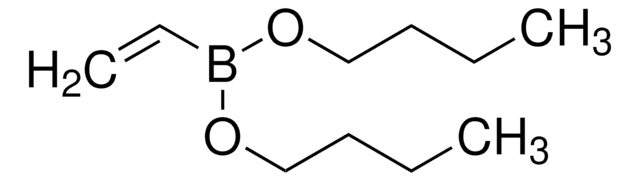
Isopropenylboronic acid pinacol ester
contains phenothiazine as stabilizer, 95%
Sinonimo/i:
2-Isopropenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(isopropenyl)-1,3,2-dioxaborolane, 4,4,5,5-Tetramethyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolane, 4,4,5,5-tetramethyl-2-(1-methylethenyl)-1,3,2-dioxaborolane
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
95%
contiene
phenothiazine as stabilizer
Indice di rifrazione
n20/D 1.4320
P. eboll.
47-49 °C/9 mbar
Densità
0.894 g/mL at 25 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(=C)B1OC(C)(C)C(C)(C)O1
InChI
1S/C9H17BO2/c1-7(2)10-11-8(3,4)9(5,6)12-10/h1H2,2-6H3
SVSUYEJKNSMKKW-UHFFFAOYSA-N
Categorie correlate
Applicazioni
- Palladium-catalyzed Suzuki-Miyaura cross-coupling processes
- Inverse-electron-demand Diels-Alder reaction
- Simmons-Smith Cyclopropanation Reaction
- Polyene cyclization
- Stereoselective aldol reactions
- Grubbs cross-metathesis reaction
- Intramolecular Suzuki-Miyaura reaction
- Stereoselective cross-metathesis
- Dipolar cycloaddition
- Iodosulfonylation
- Asymmetric conjugate addition and intramolecular hydroacylation
Reagent used in preparation of various therapeutic kinase and enzymatic inhibitors
- Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions
- Inverse-electron-demand Diels-Alder reaction
- Simmons-Smith Cyclopropanation Reaction
- Polyene cyclization
- Stereoselective aldol reactions
- Grubbs cross-metathesis reaction
- Intramolecular Suzuki-Miyaura reaction
- Stereoselective cross-metathesis
- Dipolar cycloaddition
- Iodosulfonylation
- Asymmetric conjugate addition and intramolecular hydroacylation
Reagent used in preparation of various therapeutic kinase and enzymatic inhibitors
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
107.6 °F
Punto d’infiammabilità (°C)
42 °C
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)