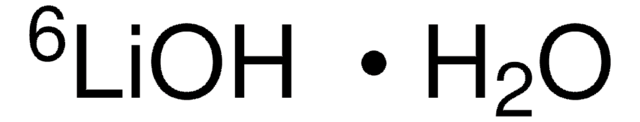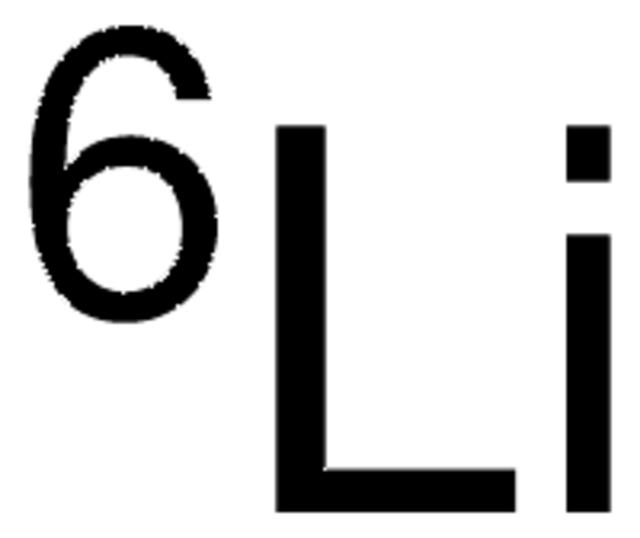601527
Lithium-7Li hydroxide monohydrate
99.9 atom % 7Li
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
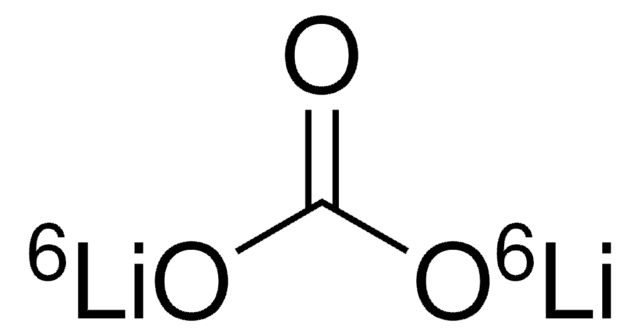
Formula condensata:
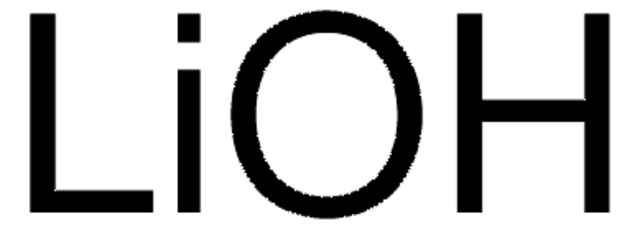
7LiOH·H2O
Numero CAS:
Peso molecolare:
42.04
Numero MDL:
Codice UNSPSC:
12352303
ID PubChem:
NACRES:
NA.12
Prodotti consigliati
Descrizione
Special high CP
Livello qualitativo
Purezza isotopica
99.9 atom % 7Li
Forma fisica
solid
Densità
1.51 g/mL at 25 °C (lit.)
Spostamento di massa
depleted
Stringa SMILE
[7Li+].O.[OH-]
InChI
1S/Li.2H2O/h;2*1H2/q+1;;/p-1/i1+0;;
GLXDVVHUTZTUQK-GLJCYROLSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Confezionamento
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
P Sivakumar et al.
Ultrasonics sonochemistry, 26, 332-339 (2015-03-10)
LiNi0.5Mn1.5O4 was synthesized as a cathode material for Li-ion batteries by a sonochemical reaction followed by annealing, and was characterized by XRD, SEM, HRTEM and Raman spectroscopy in conjunction with electrochemical measurements. Two samples were prepared by a sonochemical process
Sergej Repp et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 152, 637-644 (2015-02-25)
ZnO nanoparticles were synthesized by solid state and hydrolysis methods based on the conventional precipitation. In situ growth of ZnO nanoparticles were monitored by photoluminescence spectroscopy (PL). By the help of electron paramagnetic resonance (EPR) technique, detailed analysis of intrinsic
Martina Riesová et al.
Journal of chromatography. A, 1364, 276-288 (2014-09-13)
In this paper we determine acid dissociation constants, limiting ionic mobilities, complexation constants with β-cyclodextrin or heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin, and mobilities of resulting complexes of profens, using capillary zone electrophoresis and affinity capillary electrophoresis. Complexation parameters are determined for both neutral and
Yonghui Dong et al.
Journal of the American Society for Mass Spectrometry, 26(3), 386-389 (2015-01-18)
A significant production of gas-phase dicarboxylate dianions has been observed in standard ESI and DESI during the analysis of small organic dicarboxylic acids under moderate or highly alkaline conditions. In ESI, this can be attributed to an excess of hydroxyl
Derek Power et al.
Virology, 481, 142-150 (2015-03-18)
IFI44 is an interferon-alfa inducible protein, and is associated with infection of several viruses. However, IFI44 elicits minimal antiviral effects on these viruses, and its exact role is still unknown. Here we show that IFI44 inhibits HIV-1 replication in vitro.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.