535087
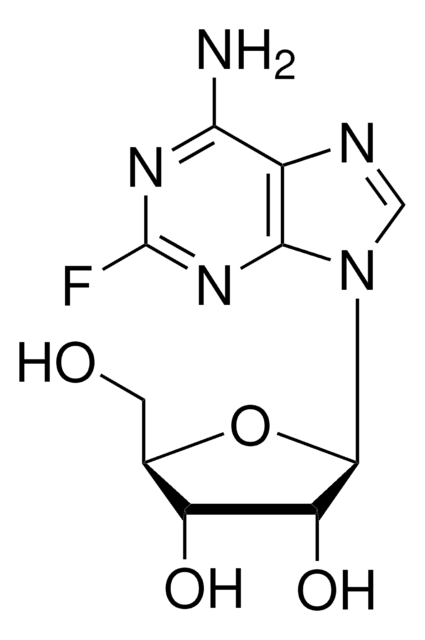
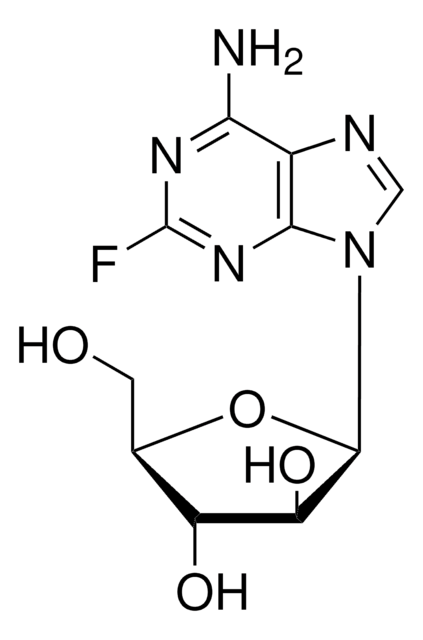
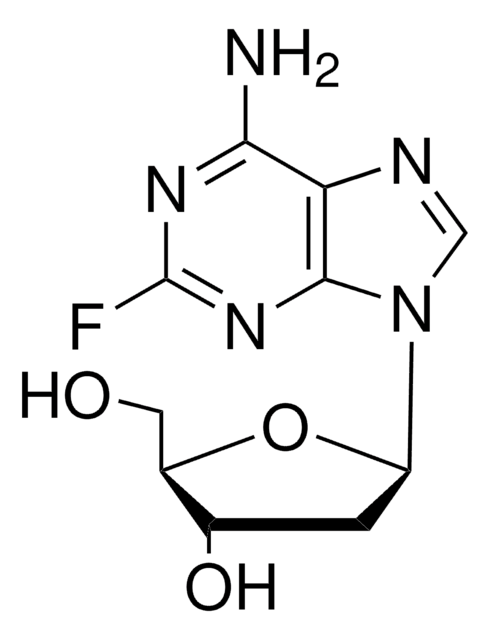
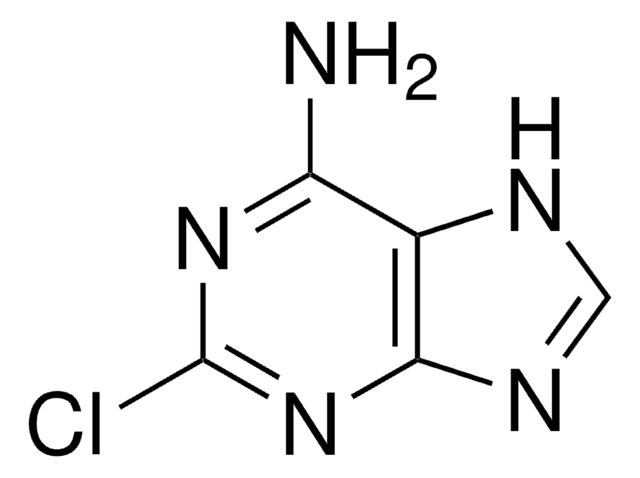
2-Fluoroadenine
96%
Sinonimo/i:
2-Fluoro-7(9)H-purin-6-ylamine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
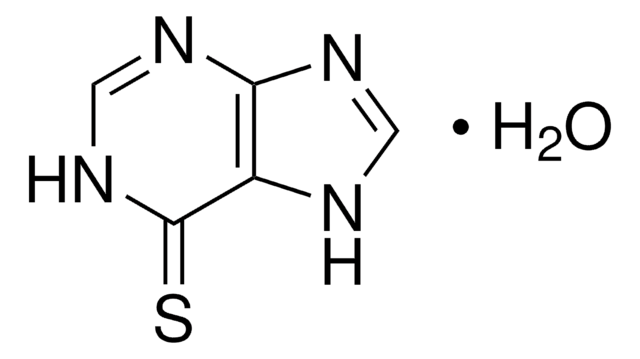
Formula empirica (notazione di Hill):
C5H4FN5
Numero CAS:
Peso molecolare:
153.12
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
96%
Punto di fusione
>350 °C (lit.)
Gruppo funzionale
fluoro
Stringa SMILE
Nc1[nH]c(F)nc2ncnc12
InChI
1S/C5H4FN5/c6-5-10-3(7)2-4(11-5)9-1-8-2/h1H,(H3,7,8,9,10,11)
WKMPTBDYDNUJLF-UHFFFAOYSA-N
Categorie correlate
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Lincoln G Scott et al.
Journal of the American Chemical Society, 126(38), 11776-11777 (2004-09-24)
The production of isotopically labeled RNA remains critical to current NMR structural studies. One approach to obtain simple NMR spectra is to label with a nucleus that is not naturally occurring in RNA. Fluorine-19 can serve as a sensitive site-specific
Yukio Kitade et al.
Nucleic acids research. Supplement (2001), (3)(3), 5-6 (2003-09-27)
Carbocyclic and acyclic nucleosides possessing 2-fluoroadenine, such as 2-fluoronoraristeromycin (6) and 2-fluoro-9-[(2S,3R)-2,3,4-trihydroxy-butyl-1-yl]adenine (8), were synthesized and their inhibitory activities against human and Plasmodium falciparum recombinant SAH hydrolase were investigated.
P Huang et al.
Biochemical pharmacology, 36(18), 2945-2950 (1987-09-15)
2-Fluoroadenine (F-Ade) is a metabolite of 9-beta-D-arabinofuranosyl-2-fluoroadenine (F-ara-A) that may be involved in the development of toxic side effects from this anticancer drug. The liberation of F-Ade from F-ara-A has been examined in different biological systems. Extracts of Escherichia coli
D Voeks et al.
Gene therapy, 9(12), 759-768 (2002-06-01)
A gene-directed enzyme pro-drug therapy (GDEPT) based on purine nucleoside phosphorylase (PNP), that converts the prodrug, fludarabine to 2-fluoroadenine, has been described, but studies are limited compared with other GDEPTs. We investigated the in vitro and in vivo efficacies of
Song Ye et al.
Nucleosides, nucleotides & nucleic acids, 22(10), 1899-1905 (2003-11-12)
A convenient synthesis of 2'-deoxy-2-fluoroadenosine from commercially available 2-fluoroadenine is described. The coupling reaction of silylated 2-fluoroadenine with phenyl 3,5-bis[O-(t-butyldimethylsilyl)]-2-deoxy-1-thio-D-erythro-pentofuranoside gave the corresponding 2-fluoro-2'-deoxyadenosine derivative (alpha/beta = 1:1) in good yield. The alpha- and beta-anomers were separated by chromatography, and
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.