528994
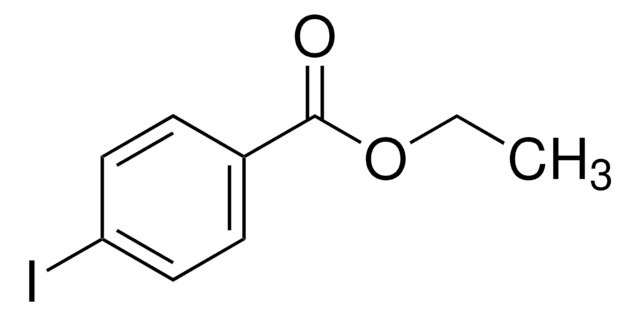
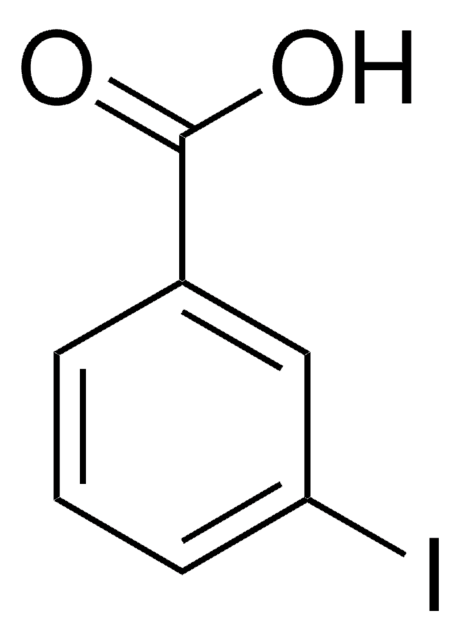
Ethyl 3-iodobenzoate
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
IC6H4CO2C2H5
Numero CAS:
Peso molecolare:
276.07
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Indice di rifrazione
n20/D 1.581 (lit.)
P. eboll.
272 °C (lit.)
Densità
1.64 g/mL at 25 °C (lit.)
Gruppo funzionale
ester
iodo
Stringa SMILE
CCOC(=O)c1cccc(I)c1
InChI
1S/C9H9IO2/c1-2-12-9(11)7-4-3-5-8(10)6-7/h3-6H,2H2,1H3
POGCXCWRMMXDAQ-UHFFFAOYSA-N
Descrizione generale
Ethyl 3-iodobenzoate is a halogenated aromatic ester. It affords arylzinc bromide via reaction with i-PrMgBr in THF, followed by reaction with ZnBr2.
Applicazioni
Ethyl 3-iodobenzoate may be used to synthesize:
- arylzinc bromide
- functionalized arylmagnesium compound
- ethyl3-phenylbenzoate
- ethyl 3-[(12-tert-butyldimethylsilyloxymethyl-1,12-dicarba-closo-dodecaboran)-1-yl]benzoate
- ethyl 3-(4-methoxy-1-methyl-2-oxo-1,2-dihydroquinolin-3-yl)benzoate
- ethyl 3-(1-methyl-2-oxo-4-phenyl-1,2-dihydroquinolin-3-yl)benzoate
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
230.0 °F - closed cup
Punto d’infiammabilità (°C)
110 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Nonpeptide Arginine Vasopressin Antagonists for Both V1A and V2 Receptors: Synthesis and Pharmacological Properties of 2-Phenyl-4'-((2, 3, 4, 5-tetrahydro-1H-1-benzazepin-1-yl) carbonyl) benzanilide Derivatives.
Matsuhisa A, et al.
Chemical & Pharmaceutical Bulletin, 45(11), 1870-1874 (1997)
Synthesis of 3, 4-Disubstituted Quinolin-2-(1H)-ones via Palladium-Catalyzed Decarboxylative Arylation Reactions.
Carrer A, et al.
Advanced Synthesis & Catalysis, 355(10), 2044-2054 (2013)
Shinya Fujii et al.
Bioorganic & medicinal chemistry, 17(1), 344-350 (2008-11-22)
A novel series of androgen receptor (AR) ligands bearing an acidic heterocycle with hydrogen-bonding ability as the terminal polar group was developed. Since most non-steroidal AR ligands so far known are structurally limited to nitro- or cyanobenzanilide as the polar
Copper catalyzed conjugate addition of highly functionalized arylmagnesium compounds to enones.
Varchi G, et al.
Tetrahedron, 56(18), 2727-2731 (2000)
Ni (II)-catalyzed cross-coupling between polyfunctional arylzinc derivatives and primary alkyl iodides.
Giovannini R and Knochel P.
Journal of the American Chemical Society, 120(43), 11186-11187 (1998)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.