47714
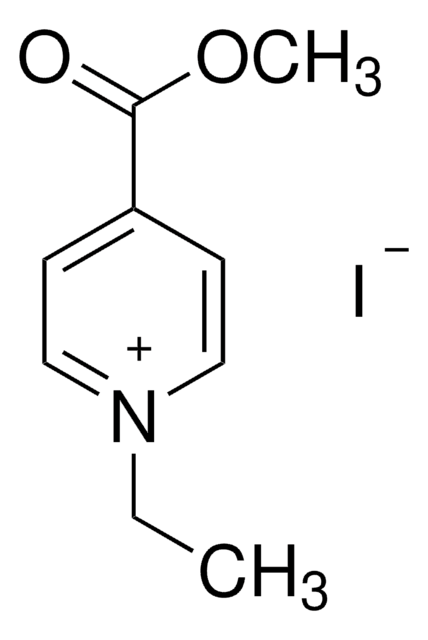
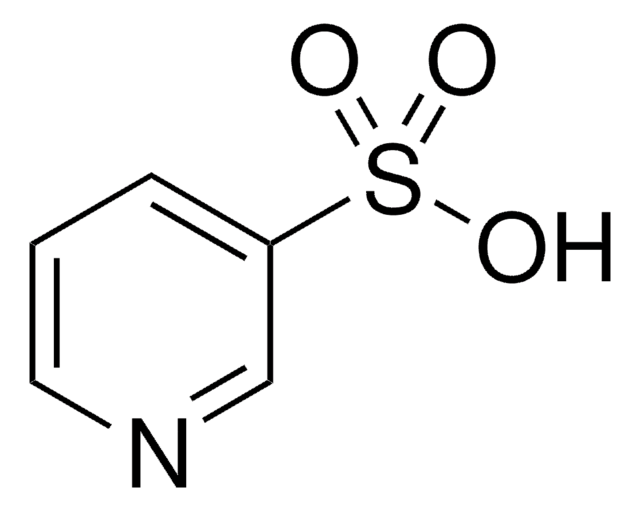
4-Formyl-1-methylpyridinium benzenesulfonate
≥95.0%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C13H13NO4S
Numero CAS:
Peso molecolare:
279.31
Beilstein:
5695963
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥95.0%
Forma fisica
solid
Impurezze
≤2.0% water
Punto di fusione
~95 °C
Gruppo funzionale
aldehyde
sulfonic acid
Stringa SMILE
[H]C(=O)c1cc[n+](C)cc1.[O-]S(=O)(=O)c2ccccc2
InChI
1S/C7H8NO.C6H6O3S/c1-8-4-2-7(6-9)3-5-8;7-10(8,9)6-4-2-1-3-5-6/h2-6H,1H3;1-5H,(H,7,8,9)/q+1;/p-1
HSVLGIFAXFDLMU-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
4-Formyl-1-methylpyridinium benzenesulfonate is a pyridinium salt widely used for the conversion of primary amines to the carbonyl compounds like aldehydes and ketones. The reaction conditions are mild, suitable for compounds with sensitive functional groups thereby providing an efficient alternative for such transformations.
Applicazioni
4-Formyl-1-methylpyridinium benzenesulfonate may be used as a reagent in the synthesis of the following:
- tetrazolic analogs of chalcones
- (+)-ferruginol
- Ecteinascidin 743
- Galipea alkaloids
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Jinchun Chen et al.
Journal of the American Chemical Society, 128(1), 87-89 (2006-01-05)
A convergent total synthesis of ecteinascidin 743 is realized from five building blocks of almost equal size. It takes 23 steps from l-3-hydroxy-4-methoxy-5-methyl phenylalanol (5) with an overall yield of 3%.
Ornella Mesenzani et al.
Bioorganic & medicinal chemistry letters, 21(2), 764-768 (2010-12-21)
In the chalcone scaffold, it is thought that the double bond is an important structural linker but it is likely not essential for the interaction with tubulin. Yet, it may be a potential site of metabolic degradation and interaction with
Zacharias Amara et al.
Natural product reports, 30(9), 1211-1225 (2013-07-31)
This review focuses on recent applications of the aza-Michael reaction in alkaloids total synthesis with a special emphasis on stereoselectivity. The report highlights achievements and challenges over the past five years and describes stereoselective intra- and inter-molecular conjugate addition of
Short syntheses of (+)-ferruginol from (+)-dehydroabietylamine.
Gonzalez MA and Perez-Guaita D.
Tetrahedron, 68(47), 9612-9615 (2012)
Mild and simple biomimetic conversion of amines to carbonyl compounds.
Buckley TF and Rapoport H
Journal of the American Chemical Society, 104(16), 4446-4450 (1982)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.