39565
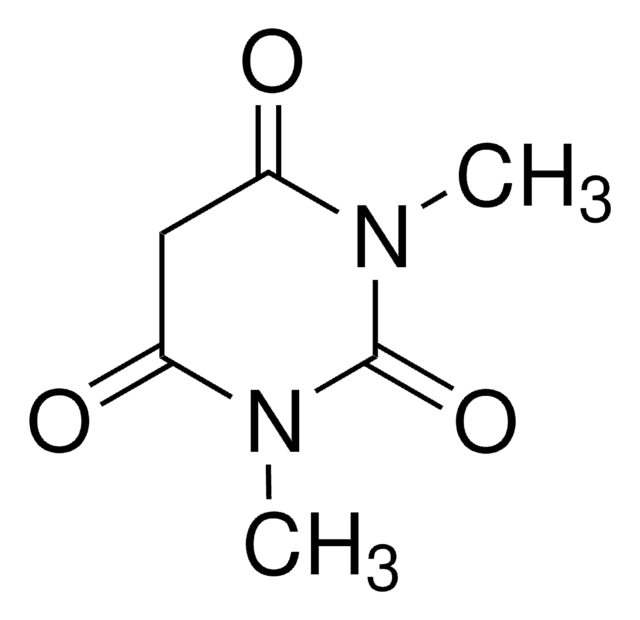
1,3-Dimethylbarbituric acid
≥99.0% (T)
Sinonimo/i:
1,3-Dimethyl-2,4,6(1H,3H,5H)-pyrimidinetrione
About This Item
Prodotti consigliati
Saggio
≥99.0% (T)
Stato
solid
Residuo alla calcinazione
≤0.1%
Punto di fusione
121-123 °C (lit.)
123-126 °C
Solubilità
hot water: soluble 0.5 g/10 mL, clear, colorless to faintly yellow
Stringa SMILE
CN1C(=O)CC(=O)N(C)C1=O
InChI
1S/C6H8N2O3/c1-7-4(9)3-5(10)8(2)6(7)11/h3H2,1-2H3
VVSASNKOFCZVES-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Enantioselective synthesis of isochromene pyrimidinedione derivatives having five stereocenters, via one-pot Michael-Knoevenagel condensation-inverse-electron-demand hetero-Diels-Alder reaction.
- Synthesis of 5-aryl-6-(alkyl- or aryl-amino)-1,3-dimethylfuro [2,3-d]pyrimidine derivatives.
- Microwave promoted indirect functionalization of alcohols, via spirocyclisation employing a sequential one-pot Ir(III)/Pd(0) catalyzed process.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.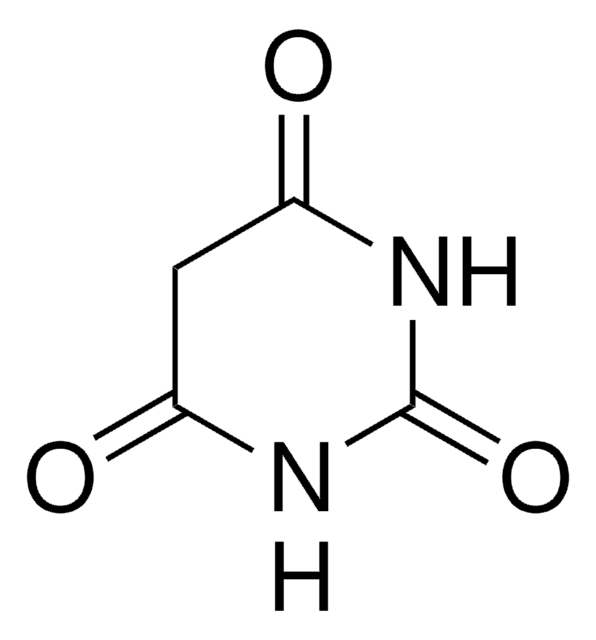


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)