456764
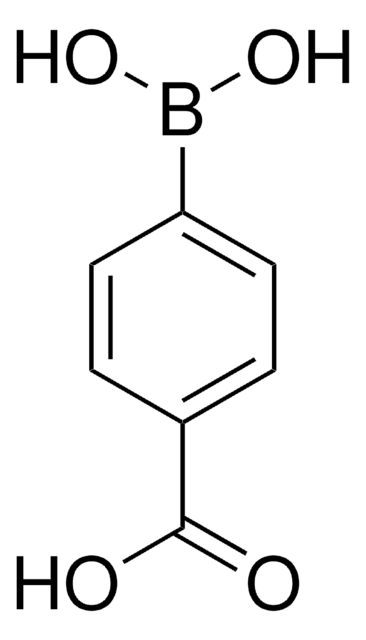
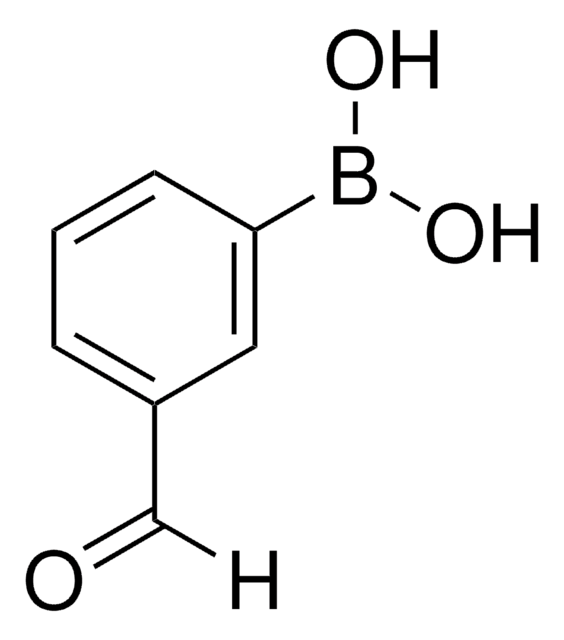
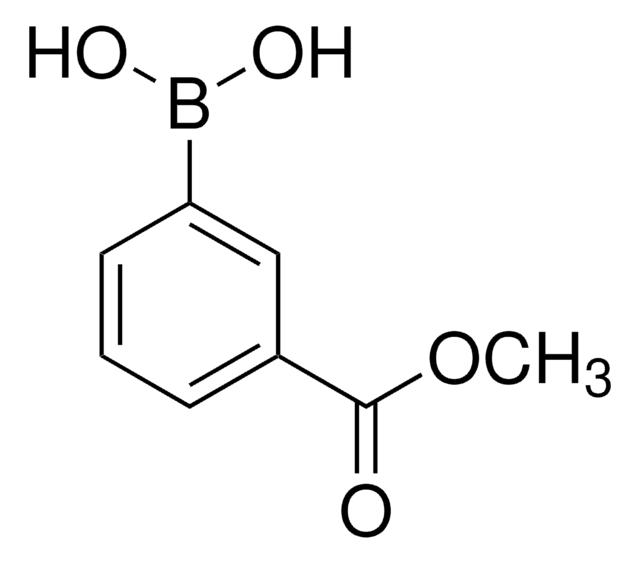
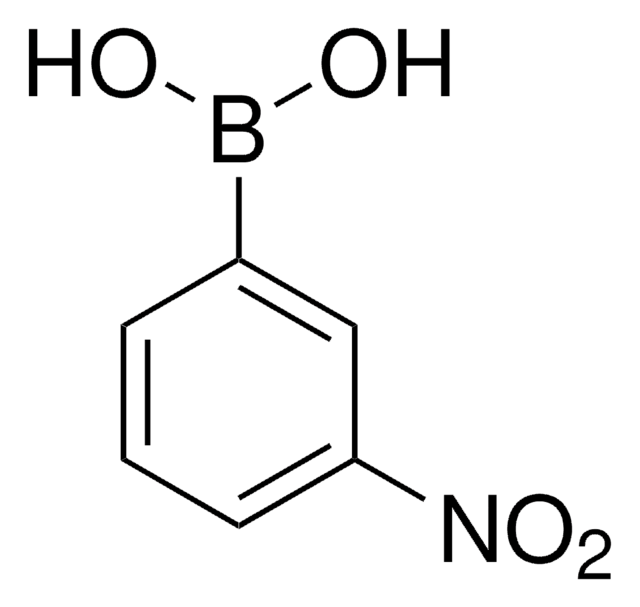
3-Carboxyphenylboronic acid
≥95%
Sinonimo/i:
μ-Carboxyphenylboronic acid, 3-(Dihydroxyborane)benzoic acid, 3-(Dihydroxyboryl)benzoic acid, 3-Boronobenzoic acid, 3-Carboxybenzeneboronic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
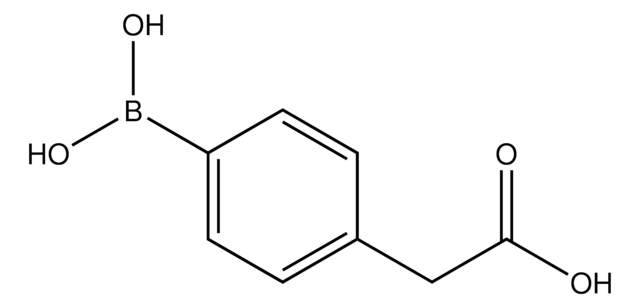
Formula condensata:
HO2CC6H4B(OH)2
Numero CAS:
Peso molecolare:
165.94
Numero MDL:
Codice UNSPSC:
12352103
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥95%
Punto di fusione
243-247 °C (lit.)
Stringa SMILE
OB(O)c1cccc(c1)C(O)=O
InChI
1S/C7H7BO4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4,11-12H,(H,9,10)
DBVFWZMQJQMJCB-UHFFFAOYSA-N
Applicazioni
3-Carboxyphenylboronic acid can be used as a substrate in the preparation of:
- Biaryl derivatives by reacting with bromoaniline through the Suzuki-Miyaura coupling reaction.
- Boronic acid-functionalized block copolymer.
- 1H-Imidazo[1,2-a]quinoxaline derivatives.
Altre note
Contains varying amounts of anhydride
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Synthesis of a phenylboronic acid-functionalized thermosensitive block copolymer and its application in separation and purification of vicinal-diol-containing compounds
Wang Y, et al.
Royal Society of Chemistry Advances, 6(85), 82309-82320 (2016)
Di Wu et al.
Acta biomaterialia, 96, 123-136 (2019-06-28)
Locoregional chemotherapy, especially using implantable hydrogel depots to sustainably deliver chemotherapeutics at tumor site, has shown great potential for improving antitumor efficacy and reducing systemic toxicity. However, the hydrogel applications are limited by some intrinsic constraints, especially the contradiction between
Jumin Yang et al.
Materials science & engineering. C, Materials for biological applications, 116, 111250-111250 (2020-08-19)
Various nanoparticles as drug delivery system provide significant improvements in the cancer treatment. However, their clinical success remains elusive in large part due to their inability to overcome both systemic and tumor tissue barriers. The nanosystems with nanoproperty-transformability (surface, size
Novel rhodamine dyes via Suzuki coupling of xanthone triflates with arylboroxins
Calitree, B. D.; Detty, M. R.
Synlett, 89-92 (2010)
New imidazo [1, 2-a] quinoxaline derivatives: synthesis and in vitro activity against human melanoma
Deleuze-Masquefa C, et al.
European Journal of Medicinal Chemistry, 44(9), 3406-3411 (2009)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.