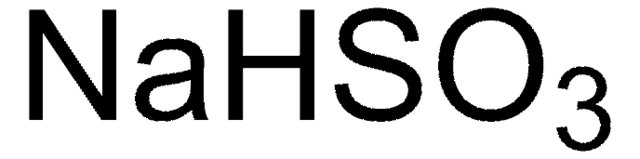
455849
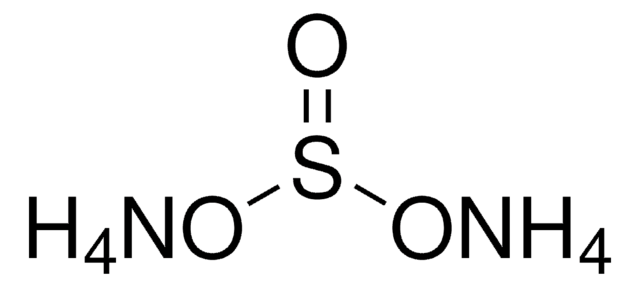
Ammonium hydrogensulfate
99.99% trace metals basis
Sinonimo/i:
Ammonium bisulfate, Ammonium sulfate monobasic
About This Item
Prodotti consigliati
Grado
for analytical purposes
Saggio
99.99% trace metals basis
Stato
crystalline
Impurezze
≤200 mg/kg Trace metallic impurities analysis (ICP)
P. ebollizione
350 °C (dec.)(lit.)
Punto di fusione
121-145 °C (lit.)
Densità
1.79 g/mL at 25 °C (lit.)
Stringa SMILE
N.OS(O)(=O)=O
InChI
1S/H3N.H2O4S/c;1-5(2,3)4/h1H3;(H2,1,2,3,4)
BIGPRXCJEDHCLP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Enhancement of Aesthetic Dental CAD-CAM Materials through Surface Etching with a Mixed Aqueous Solution of Ammonium Fluoride and Ammonium Hydrogen Sulfate - This study explores the potential of ammonium hydrogen sulfate in surface etching applications for dental materials, focusing on its low toxicity and effective etching capabilities (Y Nishizawa et al., 2024).
- Thermodynamics of ammonioalunite precipitation in ammonium aluminum sulfate solution - Investigates the thermodynamic properties of ammonium aluminum sulfate solutions, providing insights into chemical processes involving ammonium hydrogen sulfate (X Yang et al., 2020).
- Hygroscopic behavior and chemical composition evolution of internally mixed aerosols composed of oxalic acid and ammonium sulfate - Studies the hygroscopic properties of mixed aerosol particles, including those formed with ammonium hydrogen sulfate, to understand atmospheric chemical processes better (X Wang et al., 2017).
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Skin Corr. 1B
Codice della classe di stoccaggio
8B - Non-combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.