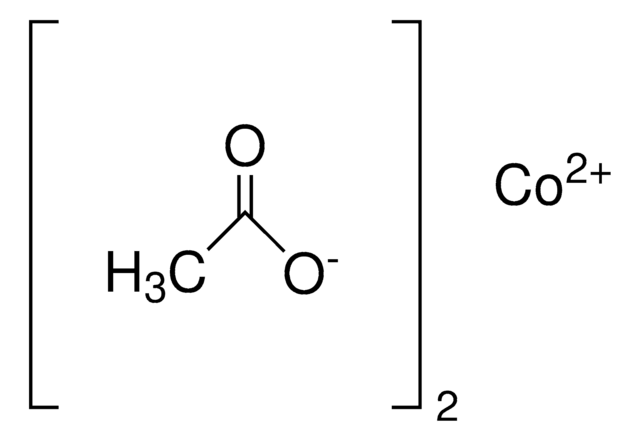
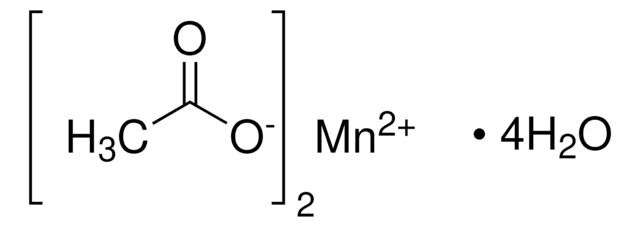
437875
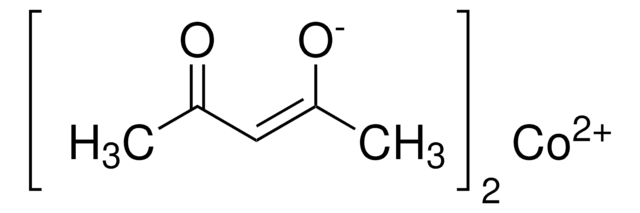
Cobalt(II) acetate tetrahydrate
99.999% trace metals basis
Sinonimo/i:
Cobaltous acetate tetrahydrate
About This Item
Prodotti consigliati
Saggio
99.999% trace metals basis
Stato
powder and chunks
Impiego in reazioni chimiche
core: cobalt
Stringa SMILE
[H]O[H].[H]O[H].[H]O[H].[H]O[H].CC(=O)O[Co]OC(C)=O
InChI
1S/2C2H4O2.Co.4H2O/c2*1-2(3)4;;;;;/h2*1H3,(H,3,4);;4*1H2/q;;+2;;;;/p-2
ZBYYWKJVSFHYJL-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
From Form to Function: Molding Porous Materials in Three Dimensions by Colloidal Crystal Templating
Nanostructured Materials Through Ultrasonic Spray Pyrolysis
Advances in materials have often been led by the development of new synthetic methods that provide control over size, morphology and structure. The preparation of materials in a scalable and continuous manner is critical when development moves beyond lab-scale quantities.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.