244066
Nickel(II) acetate tetrahydrate
98%
Sinonimo/i:
Acetic acid nickel(II) salt
About This Item
Prodotti consigliati
Saggio
98%
Stato
powder
Impiego in reazioni chimiche
core: nickel
reagent type: catalyst
Densità
1.798 g/mL at 25 °C (lit.)
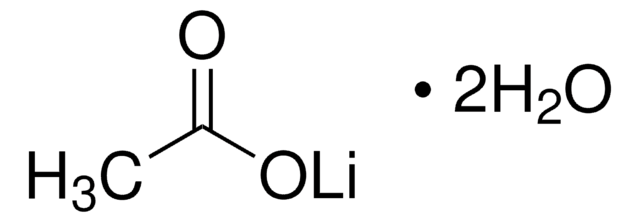
Stringa SMILE
[H]O[H].[H]O[H].[H]O[H].[H]O[H].CC(=O)O[Ni]OC(C)=O
InChI
1S/2C2H4O2.Ni.4H2O/c2*1-2(3)4;;;;;/h2*1H3,(H,3,4);;4*1H2/q;;+2;;;;/p-2
OINIXPNQKAZCRL-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Used as a precursor material in the hydrothermal synthesis of nanostructured nickel diselenide (NiSe2).
- Used as a precursor in the synthesis of the salamo-based dinuclear nickel(II) complex.
- Used as a precursor material in the synthesis of nickel oxide (NiO) via sol-gel method. Nickel oxide can be tailored for various applications, including electrochromic devices, gas sensors, and lithium-ion batteries.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1 - STOT RE 1
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.