436798
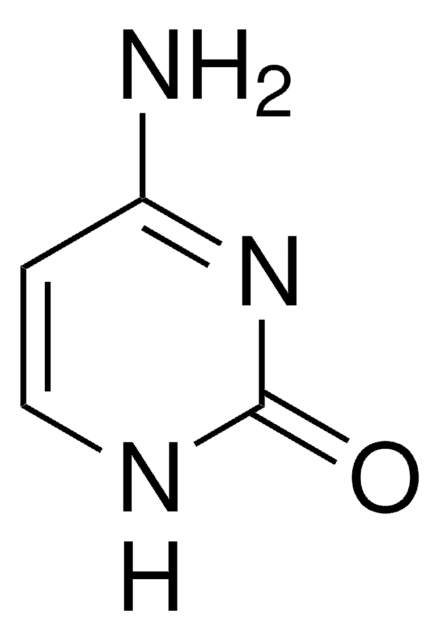
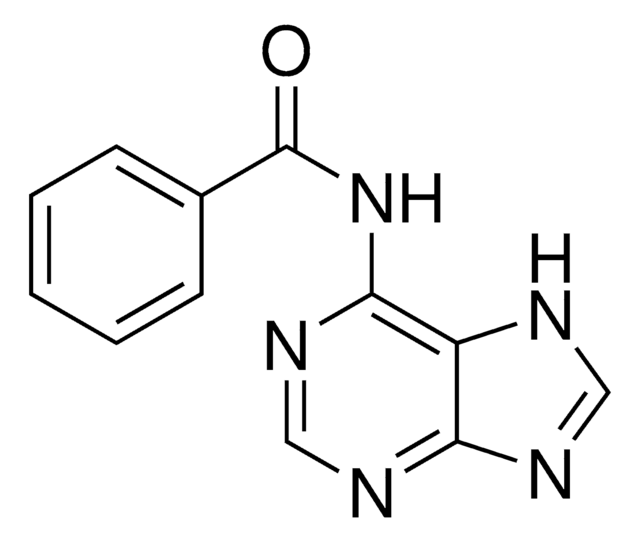
N4-Benzoylcytosine
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C11H9N3O2
Numero CAS:
Peso molecolare:
215.21
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Punto di fusione
>300 °C (dec.) (lit.)
Gruppo funzionale
amide
phenyl
Stringa SMILE
O=C1NC=CC(NC(=O)c2ccccc2)=N1
InChI
1S/C11H9N3O2/c15-10(8-4-2-1-3-5-8)13-9-6-7-12-11(16)14-9/h1-7H,(H2,12,13,14,15,16)
XBDUZBHKKUFFRH-UHFFFAOYSA-N
Descrizione generale
N4-Benzoylcytosine is an amide and its anti-microbial activity against pathogenic microorganisms has been studied using the Disk Diffusion and the Pour Plate method. It can be synthesized via the condensation of benzoyl chloride with cytosine.
Applicazioni
N4-Benzoylcytosine may be employed for the following syntheses:
- 3′-C-ethynyl and 3′-C-(1,4-disubstituted-1,2,3-triazolo) double-headed pyranonucleosides
- 2′-C-methyl-4′-thiocytidine, via the Pummerer reaction
- 2′-fluorinated L-nucleoside analogs
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Christos Kiritsis et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 8(3), 320-329 (2012-04-26)
A novel series of 3'-C-ethynyl and 3'-C-(1,4-disubstituted-1,2,3-triazolo) double-headed pyranonucleosides has been designed and synthesized. Reaction of 3-keto glucoside 1 with ethynyl magnesium bromide gave the desired precursor 3-C-ethynyl-1,2:5,6-di-O-isopropylidene-α-D-allofuranose (2). Hydrolysis followed by acetylation led to the 1,2,4,6-tetra-O-acetyl-3-C-ethynyl-β-D-allopyranose (3). Compound 3
Daisuke Kaga et al.
Nucleosides, nucleotides & nucleic acids, 24(10-12), 1789-1800 (2006-01-28)
The synthesis of 2'-C-methyl-4'-thiocytidine (16) is described. Since the 2'-keto-4'-thiocytidine derivative 2beta unexpectedly isomerized to 2alpha and the methylation of 2beta proceeded predominantly from the less hindered alpha-face to give 7, the desired product 16 was synthesized via the Pummerer
Antimicrobial activity of amide, N4-benzoylcytosine.
Jagessar RC and Gomathinayagam S.
Journal of Pharmacy and Clinical Sciences, 1, 12-19 (2011)
K Lee et al.
Journal of medicinal chemistry, 42(7), 1320-1328 (1999-04-10)
The synthesis of L-nucleoside analogues containing 2'-vinylic fluoride was accomplished by direct condensation method, and their anti-HIV and anti-HBV activities were evaluated in vitro. The key intermediate 8, the sugar moiety of our target compounds, was prepared from 1,2-O-isopropylidene-L-glyceraldehyde via
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.