405418
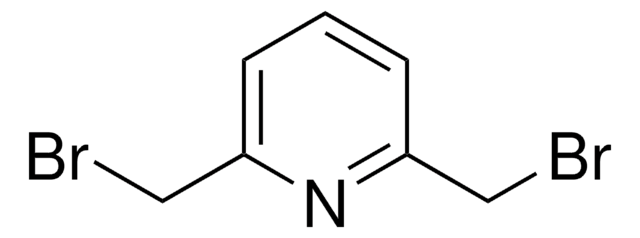
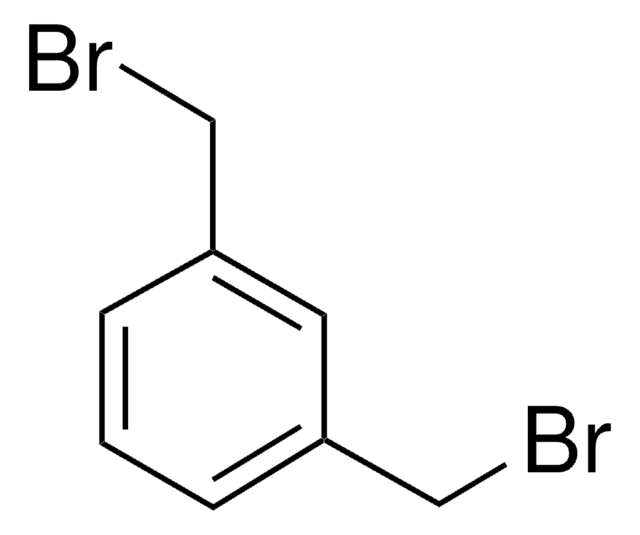
2,6-Bis(chloromethyl)pyridine
99%
Sinonimo/i:
α,α′-Dichloro-2,6-lutidine
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C7H7Cl2N
Numero CAS:
Peso molecolare:
176.04
Beilstein:
116355
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
99%
Stato
solid
Punto di fusione
73-78 °C (lit.)
Gruppo funzionale
chloro
Stringa SMILE
ClCc1cccc(CCl)n1
InChI
1S/C7H7Cl2N/c8-4-6-2-1-3-7(5-9)10-6/h1-3H,4-5H2
IWQNFYRJSVJWQA-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
2,6-Bis(chloromethyl)pyridine is a heterocyclic building block for the synthesis of a variety of pyridine derivatives. It coordinates with metal ions through N-atom to form complexes. The conformational flexibility of the bromomethyl arms makes it an ideal choice for the generation of macrocycles. 2,6-bis(chloromethyl)pyridine crystals are monoclinic with space group P21/c. Its synthesis has been reported. The FT-IR and FT-Raman spectra of 2,6-bis(chloromethyl)pyridine (BCMP) have been recorded in the regions 4000-400cm-1 and 3500-100cm-1, respectively.
Applicazioni
2,6-Bis(chloromethyl)pyridine may be used in the following studies:
- Synthesis of a sensitive fluorescent chemosensor for Hg2+, composed of two aminonaphthalimide fluorophores and a receptor of 2,6-bis(aminomethyl)pyridine.
- Preparation of carbene pincer ligands, required for the preparation of palladium pincer carbene complex.
- Synthesis of 2-(di-tert-butylphosphinomethyl)-6-diethylaminomethyl)pyridine, PNN ligand.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Iron (II) complexes based on electron-rich, bulky PNN-and PNP-type ligands.
Zhang J, et al.
Inorgorganica Chimica Acta, 359(6), 1955-1960 (2006)
Xiangfeng Guo et al.
Journal of the American Chemical Society, 126(8), 2272-2273 (2004-02-26)
A selective and sensitive fluorescent chemosensor for Hg2+, which was composed of two aminonaphthalimide fluorophores and a receptor of 2,6-bis(aminomethyl)pyridine, was synthesized through the reaction of 2,6-bis(chloromethyl)pyridine and N-[2-(2-hydroxyethoxy)ethyl]-4-piperazino-1,8-naphthalimide. The chemosensor showed an about 17-fold increase in fluorescence quantum yield
Tridentate carbene CCC and CNC pincer palladium (II) complexes: structure, fluxionality, and catalytic activity.
Grundemann S, et al.
Organometallics, 20(25), 5485-5488 (2001)
V Balachandran et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 97, 1023-1032 (2012-08-29)
The FT-IR and FT-Raman spectra of 2,6-Bis(chloromethyl)pyridine (BCMP) have been recorded in the regions 4000-400 cm(-1) and 3500-100 cm(-1), respectively. The total energy calculations of BCMP were tried for the possible rotational isomers. The molecular structure, geometry optimization, vibrational frequencies
An efficient synthesis of 2,6-bis(chloromethyl)pyridine and a [5.5](2,6) pyridinophane disulfite.
Rezzonico B and Grignon-Dubois M.
J. Chem. Res. Synop., 4, 142-143 (1994)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.