404276
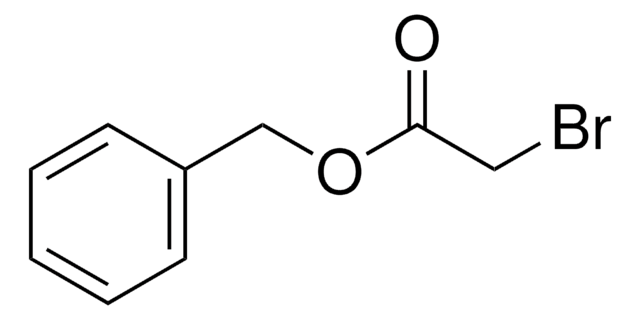
Phenyl bromoacetate
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
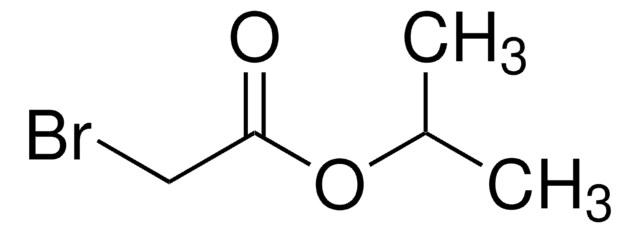
Formula condensata:
BrCH2CO2C6H5
Numero CAS:
Peso molecolare:
215.04
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Stato
solid
P. ebollizione
134 °C/15 mmHg (lit.)
Punto di fusione
31-33 °C (lit.)
Densità
1.508 g/mL at 25 °C (lit.)
Gruppo funzionale
bromo
ester
phenoxy
Stringa SMILE
BrCC(=O)Oc1ccccc1
InChI
1S/C8H7BrO2/c9-6-8(10)11-7-4-2-1-3-5-7/h1-5H,6H2
UEWYUCGVQMZMGY-UHFFFAOYSA-N
Descrizione generale
Phenyl bromoacetate is an aromatic ester.
Applicazioni
Phenyl bromoacetate may be employed as alkylation reagent in the preparation of 2-(phenoxycarbonyl)methyl triazoles. It may be used in the synthesis of the A-ring of cylindrospermopsin. It may be used in the synthesis of the following 4-thiazolidinones:
- 2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene]hydrazinyl}-1,3-thiazolidin-4-one
- 2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene]hydrazinyl}-3-methyl-1,3-thiazolidin-4-one
- 3-ethyl-2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene]hydrazinyl}-1,3-thiazolidin-4-one
- 2-{2-[1-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)ethylidene] hydrazinyl}-3-phenyl-1,3-thiazolidin-4-one
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Synthesis and Antimicrobial Activity Evaluation of Novel 4-Thiazolidinones Containing a Pyrone Moiety.
Nechak R, et al.
Synthetic Communications, 1-11 (2014)
Robert M Williams et al.
ACS symposium series. American Chemical Society, 1009, 420-442 (2010-06-22)
We report the application of diphenyloxazinone glycinate chiral templates to asymmetric syntheses of cylindrospermospin, 7-epi-cylindrospermopsin, 7-deoxycylindrospermopsin, and spirotryprostatins A and B. Synthetic studies toward quinine, nakadomarin A, and palau'amine using these templates are also described.
Duen-Ren Hou et al.
Bioorganic & medicinal chemistry letters, 19(3), 1022-1025 (2008-12-20)
This letter reports the new entry of novel 1,2,3-triazole derivatives as CB1 receptor antagonists. The design, synthesis and biological evaluation of N1 and N2 substituted 1,2,3-trizoles are described. The N2 substituted, symmetrical 1,2,3-triazoles are more potent ligands than the unsymmetrical
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.