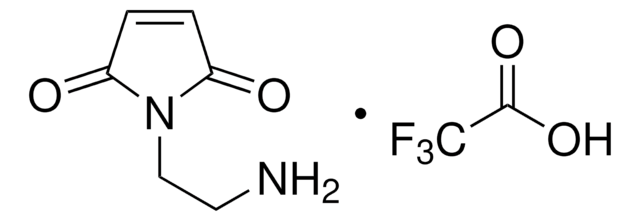
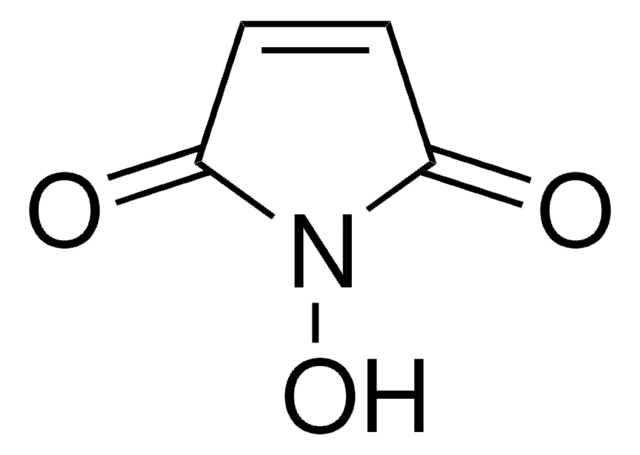
394815
N-Maleoyl-β-alanine
97%
Sinonimo/i:
3-Maleimidopropionic acid, N-(2-Carboxyethyl)maleimide
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
97%
Stato
powder
Punteggio alternativa più verde
old score: 18
new score: 7
Find out more about DOZN™ Scoring
Caratteristiche più verdi
Less Hazardous Chemical Syntheses
Safer Solvents and Auxiliaries
Inherently Safer Chemistry for Accident Prevention
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Punto di fusione
103-106 °C (lit.)
Gruppo funzionale
carboxylic acid
imide
maleimide
Categoria alternativa più verde
Temperatura di conservazione
2-8°C
Stringa SMILE
OC(=O)CCN1C(=O)C=CC1=O
InChI
1S/C7H7NO4/c9-5-1-2-6(10)8(5)4-3-7(11)12/h1-2H,3-4H2,(H,11,12)
IUTPJBLLJJNPAJ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- To decrease the biotin binding affinity of Avd(S16C) (avidin with a single point mutation S16C).
- As a side chain reactive agent to modify tryptic peptides that result in mass shifts indicating the presence of cysteine residues.
- To preblock Xenopus laevis oocytes for exposed cysteines used as an expression system in the study of conformational changes in cASIC1a Receptors.
- As a protective agent for keratin fiber in high temperature process.
- As a non-cleavable maleimido moiety during the synthesis of tetrawalled molecular umbrella-octaarginine conjugates.
- Synthesis of organotin carboxylates of N-Maleoyl-β-alanine.
- To functionalize the gold surfaces to interact with cysteine-modified peptide.
- Preparation of cross-linked dextran–poly(ethylene glycol) hydrogel substrate.
Confezionamento
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 394815-1G | 4061831982497 |
| 394815-250MG | 4061831820126 |
| 394815-50MG |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.