365920
Diethyl phosphite
technical grade, 94%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
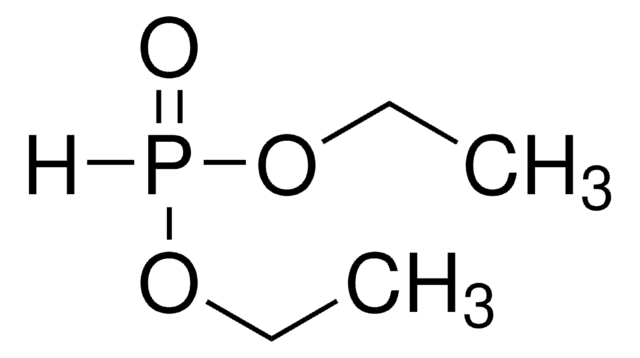
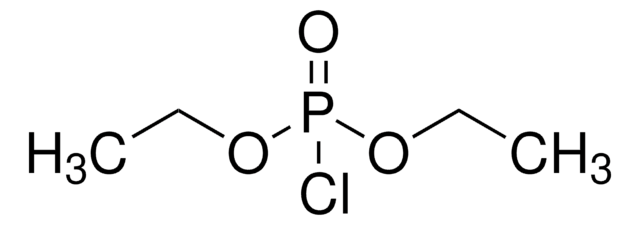
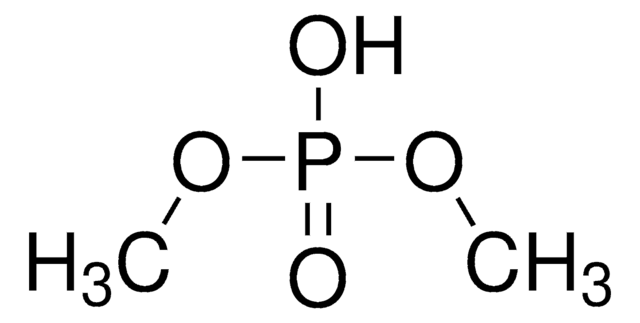
Formula condensata:
(C2H5O)2P(O)H
Numero CAS:
Peso molecolare:
138.10
Beilstein:
605759
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Grado
technical grade
Livello qualitativo
Saggio
94%
Stato
liquid
Indice di rifrazione
n20/D 1.407 (lit.)
P. ebollizione
50-51 °C/2 mmHg (lit.)
Densità
1.072 g/mL at 25 °C (lit.)
Stringa SMILE
[H]P(=O)(OCC)OCC
InChI
1S/C4H11O3P/c1-3-6-8(5)7-4-2/h8H,3-4H2,1-2H3
MJUJXFBTEFXVKU-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Diethyl phosphite is reported to be chemical warfare agent (CWA) simulant, simulant for nerve agents sarin, soman, tabun, and VX.
Applicazioni
Diethyl phosphite may be used to prepare the nickel chloride-diethyl phosphite system, efficient catalyst for the cross-coupling reaction between various functionalized arylzinc halides and aryl bromides, triflates and activated chlorides. It may be used:
- in the stereoselective synthesis of β-fluorinated alkylphosphonates
- in the synthesis of homoallylic bromide
- in the synthesis of series of carbazole-based α-aminophosphonates
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Dam. 1 - Skin Sens. 1B
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
179.6 °F - closed cup
Punto d’infiammabilità (°C)
82 °C - closed cup
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Wenke Qi et al.
The Journal of organic chemistry, 78(12), 5918-5924 (2013-05-22)
Cyclopropyl Grignard reagents react with carbonyl compounds in the presence of diethyl phosphite to give homoallylic bromides. The reaction is effectively carried out under mild conditions in a one-pot fashion with moderate to good yields.
Adam M Graichen et al.
Journal of the American Society for Mass Spectrometry, 24(6), 917-925 (2013-03-28)
The gas-phase reactions of a series of coordinatively unsaturated [Ni(L)n](y+) complexes, where L is a nitrogen-containing ligand, with chemical warfare agent (CWA) simulants in a miniature rectilinear ion trap mass spectrometer were investigated as part of a new approach to
Anil Kumar Mungara et al.
Chemical & pharmaceutical bulletin, 60(12), 1531-1537 (2012-09-19)
A novel series of carbazole-based α-aminophosphonates were synthesized by three component coupling of 6-bromo-9-ethyl-9H-carbazole-3-carbaldehyde, amine and diethyl phosphite using polyethylene glycol (PEG-400) as a green reaction media. The antiproliferative activity of these molecules was evaluated against three cancer cell lines.
Andrei Gavryushin et al.
Organic letters, 7(22), 4871-4874 (2005-10-21)
[reaction: see text] The combination of diethyl phosphite and DMAP as ligands for nickel in an 8:1 THF-N-ethylpyrrolidinone (NEP) mixture allows a very efficient cross-coupling reaction to be performed between various functionalized arylzinc halides and aryl bromides, triflates and activated
Chengwei Zhang et al.
Journal of the American Chemical Society, 135(38), 14082-14085 (2013-09-13)
We report herein a mild and catalytic phosphonofluorination of unactivated alkenes. With catalysis by AgNO3, the condensation of various unactivated alkenes with diethyl phosphite and Selectfluor reagent in CH2Cl2/H2O/HOAc at 40 °C led to the efficient synthesis of β-fluorinated alkylphosphonates
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.