296783
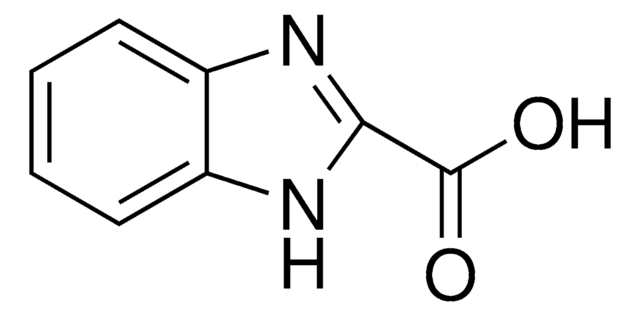
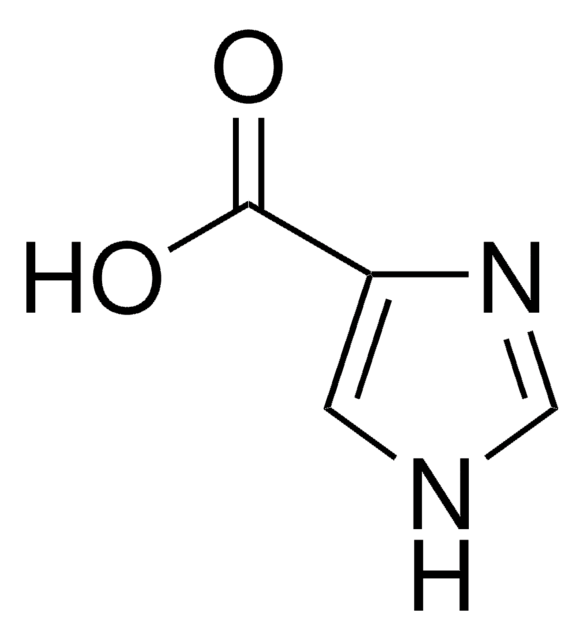
5-Benzimidazolecarboxylic acid
96%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C8H6N2O2
Numero CAS:
Peso molecolare:
162.15
Numero MDL:
Codice UNSPSC:
12352100
eCl@ss:
32151902
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
96%
Punto di fusione
>300 °C (lit.)
Gruppo funzionale
carboxylic acid
Stringa SMILE
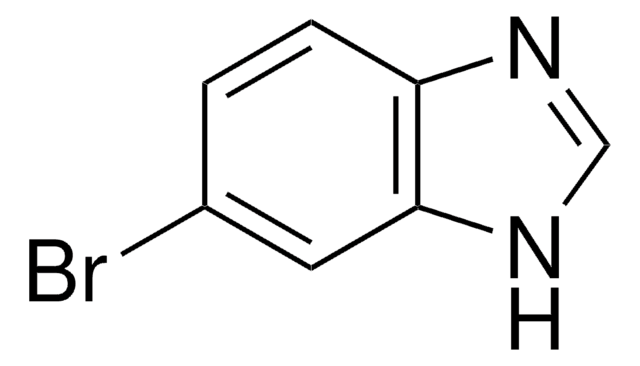
OC(=O)c1ccc2[nH]cnc2c1
InChI
1S/C8H6N2O2/c11-8(12)5-1-2-6-7(3-5)10-4-9-6/h1-4H,(H,9,10)(H,11,12)
COYPLDIXZODDDL-UHFFFAOYSA-N
Descrizione generale
Drug-specific monoclonal antibodies were produced against the very small drug hapten, 5-benzimidazolecarboxylic acid.
Applicazioni
5-Benzimidazolecarboxylic acid has been used in the preparation of:
- 1H-benzoimidazole-5-carboxylic acid benzotriazol-1-yl ester
- piperidin-1-yl(1-m-tolyl-1H-benzo[d]imidazol-5-yl)methanone
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Ajit Jadhav et al.
Probe Reports from the NIH Molecular Libraries Program, 2010 Mar 4 (Updated 2011 Mar 11) (2011-07-08)
15-hydroxyprostaglandin dehydrogenase (15-PGDH; HPGD) is the key enzyme for the inactivation of prostaglandins, and thus regulates processes such as inflammation or proliferation. The anabolic pathways of prostaglandins are well-characterized, especially with respect to regulation of the cyclooxygenase (COX) enzymes. In
E Moran et al.
Journal of immunological methods, 271(1-2), 65-75 (2002-11-26)
Drug-specific monoclonal antibodies (MAbs) were produced against the very small drug hapten (162.15 Da), 5-benzimidazolecarboxylic acid, an analogue of 2-(4-Thiazolyl)benzimidazole (TBZ) but lacking the thiol group. TBZ is widely used as a broad-spectrum anthelmintic in various animal species and humans
E S Medlock et al.
The Anatomical record, 207(1), 31-41 (1983-09-01)
Mouse fetal liver was studied ultrastructurally to identify and characterize the developing hepatic parenchyma or prehepatocyte which may be responsible for producing the liver hemopoietic environment. It was observed that as the liver develops, there is close association of endodermal
Xiaofei Chen et al.
Biomaterials science, 3(6), 870-878 (2015-07-30)
Herein, hyperbranched poly(ethylene glycol)-based supramolecular nanoparticles with pH-sensitive properties were designed and used for targeted drug delivery. Via host-guest recognition between benzimidazole anchored poly(ethylene glycol)-hyperbranched polyglycerol (PEG-HPG-BM) and folic acid modified CD (FA-CD), targeted supramolecular nanoparticles (TSNs) were fabricated. At
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.