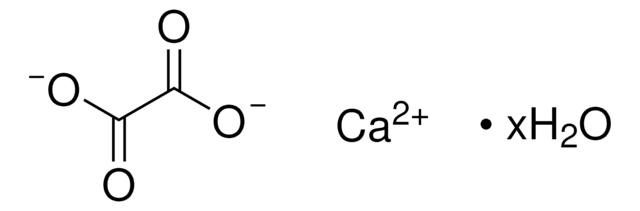289841
Calcium oxalate hydrate
Sinonimo/i:
Calcium ethanedioate hydrate
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C2CaO4 · xH2O
Numero CAS:
Peso molecolare:
128.10 (anhydrous basis)
Numero CE:
Numero MDL:
Codice UNSPSC:
12352302
ID PubChem:
NACRES:
NA.23
Prodotti consigliati
Forma fisica
powder
Concentrazione
57.0-62.0% (by KMnO4, %C2O4, titration)
Stringa SMILE
O.O=C1O[Ca]OC1=O
InChI
1S/C2H2O4.Ca.H2O/c3-1(4)2(5)6;;/h(H,3,4)(H,5,6);;1H2/q;+2;/p-2
LQHWSGSWNOHVHO-UHFFFAOYSA-L
Applicazioni
- Local concentration controls the hydrate phase of calcium oxalate.: This research demonstrates how adjusting the titration rate of calcium solution and stirring speed can influence the formation of different hydrate phases of calcium oxalate. Such control is critical for applications requiring specific crystal forms, such as in bioceramics and industrial processes (Wang et al., 2024).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
D Sujatha et al.
International braz j urol : official journal of the Brazilian Society of Urology, 41(3), 511-520 (2015-07-23)
Urolithiasis is a common urological disorder responsible for serious human affliction and cost to the society with a high recurrence rate. The aim of the present study was to systematically evaluate the phlorotannin rich extract of Sargassum wightii using suitable
Xin Wang et al.
International journal of molecular medicine, 45(2), 375-384 (2020-01-03)
MicroRNAs (miRNAs or miRs) are critical regulators in various diseases. In the current study, the role of miR‑30c‑5p in the formation of sodium oxalate‑induced kidney stones was investigated. For this purpose, human renal tubular epithelial cells (HK‑2 cells) were incubated
J Tonannavar et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 154, 20-26 (2015-10-27)
We present in this paper accurate and reliable Raman and IR spectral identification of mineral constituents in nine samples of renal calculi (kidney stones) removed from patients suffering from nephrolithiasis. The identified mineral components include Calcium Oxalate Monohydrate (COM, whewellite)
A Schmalenberger et al.
Scientific reports, 5, 12187-12187 (2015-07-23)
Trees and their associated rhizosphere organisms play a major role in mineral weathering driving calcium fluxes from the continents to the oceans that ultimately control long-term atmospheric CO2 and climate through the geochemical carbon cycle. Photosynthate allocation to tree roots
Daniel Bravo et al.
Archives of microbiology, 197(1), 65-77 (2014-11-10)
The oxalate-carbonate pathway (OCP) is a biogeochemical set of reactions that involves the conversion of atmospheric CO2 fixed by plants into biomass and, after the biological recycling of calcium oxalate by fungi and bacteria, into calcium carbonate in terrestrial environments.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.