254037
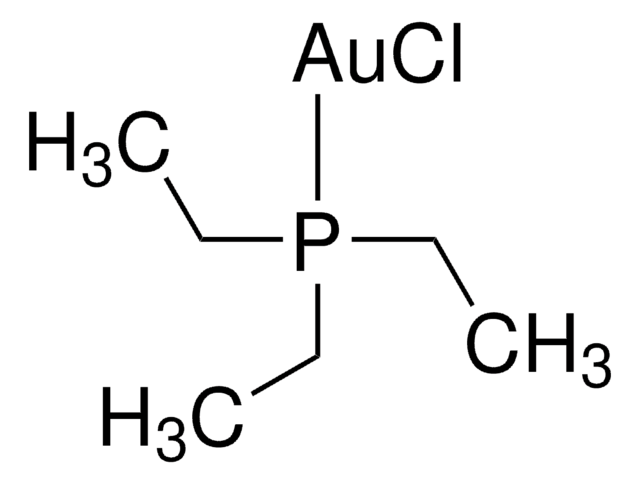
Chloro(triphenylphosphine)gold(I)
≥99.9% trace metals basis
Sinonimo/i:
(Ph3P)AuCl, Triphenylphosphinegold(I) chloride
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥99.9% trace metals basis
Stato
solid
Impiego in reazioni chimiche
core: gold
reagent type: catalyst
Stringa SMILE
Cl[Au].c1ccc(cc1)P(c2ccccc2)c3ccccc3
InChI
1S/C18H15P.Au.ClH/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;/h1-15H;;1H/q;+1;/p-1
IFPWCRBNZXUWGC-UHFFFAOYSA-M
Categorie correlate
Applicazioni
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
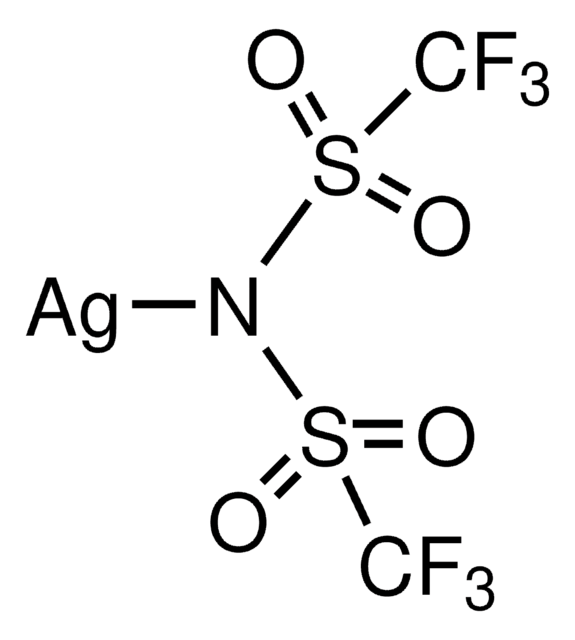
The importance of selectively fluorinating compounds in medicinal chemistry, biology, and organic synthesis is well appreciated and provides a major impetus to the discovery of new and mild fluorinating agents that can operate safely and efficiently.
We are proud to offer a treasure-trove of gold precatalysts and silver salts, as well as an extensive portfolio of unsaturated building blocks to accelerate your research success in this exciting field.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 254037-500MG | 4061825982762 |
| 254037-5G | 4061833545676 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.
![Chloro[tris(para-trifluoromethylphenyl)phosphine]gold(I) 99%](/deepweb/assets/sigmaaldrich/product/structures/250/453/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e/640/f96e05ee-0d9c-46a0-b0f5-818f89e15a2e.png)
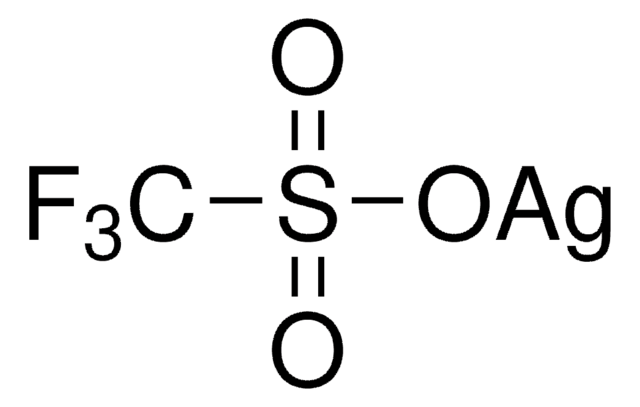
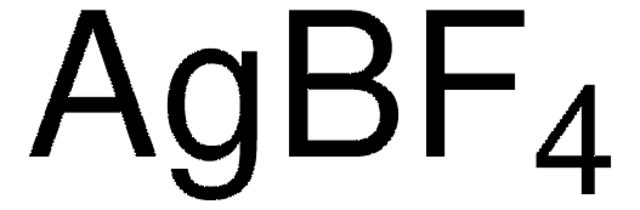

![[1,3-Bis(2,6-diisopropylphenyl-imidazol-2-ylidene]gold(I)chloride Umicore](/deepweb/assets/sigmaaldrich/product/structures/186/572/1f89dfca-fb52-46a2-9c9d-96db67c22883/640/1f89dfca-fb52-46a2-9c9d-96db67c22883.png)
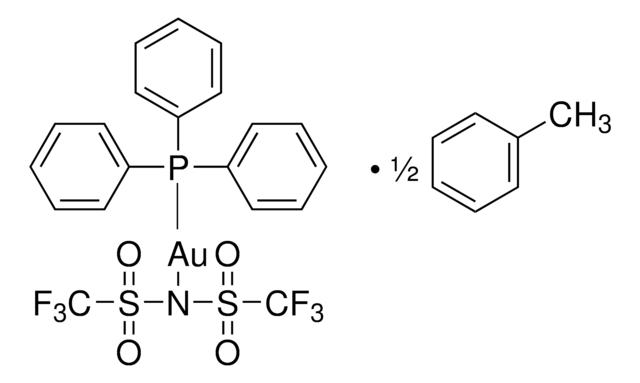

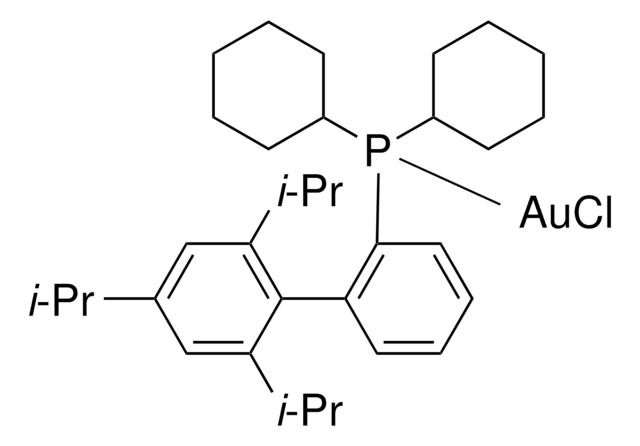
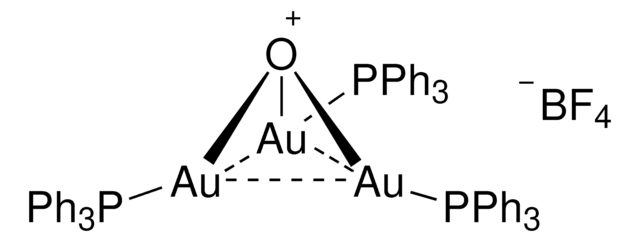
![Chloro[tris(2,4-di-tert-butylphenyl)phosphite]gold](/deepweb/assets/sigmaaldrich/product/structures/386/294/6df0db46-002b-4599-ad6c-451c419a3fc5/640/6df0db46-002b-4599-ad6c-451c419a3fc5.png)
![(Acetonitrile)[(2-biphenyl)di-tert-butylphosphine]gold(I) hexafluoroantimonate](/deepweb/assets/sigmaaldrich/product/structures/216/222/abe04540-8e4f-41fc-bcb8-2e1e0f25c8b9/640/abe04540-8e4f-41fc-bcb8-2e1e0f25c8b9.png)