241989
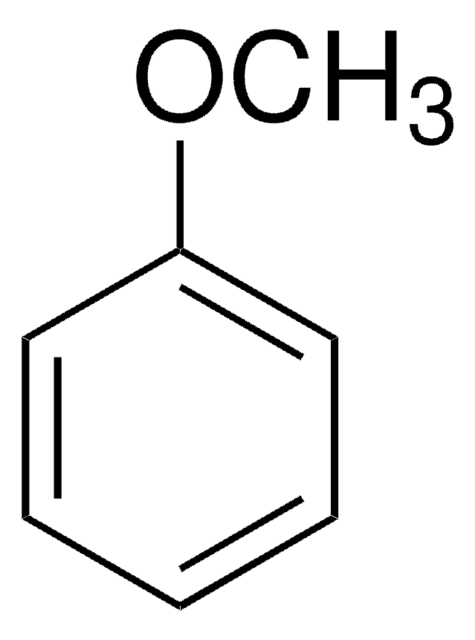
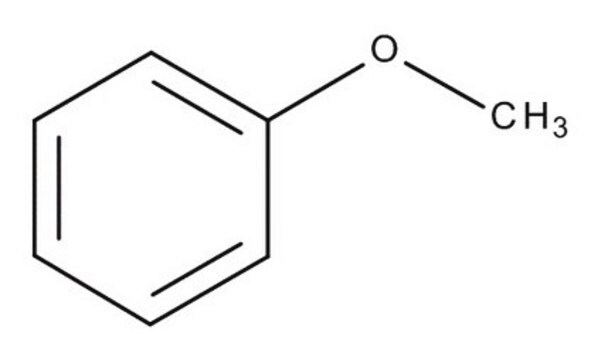
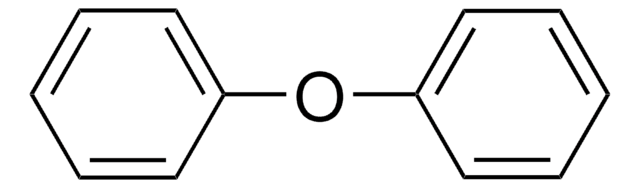
Ethoxybenzene
99%
Sinonimo/i:
Phenetole, Ethoxybenzene, Ethyl phenyl ether
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5OC2H5
Numero CAS:
Peso molecolare:
122.16
Beilstein:
636270
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
Forma fisica
liquid
Indice di rifrazione
n20/D 1.507 (lit.)
P. eboll.
169-170 °C (lit.)
Punto di fusione
−30 °C (lit.)
Solubilità
alcohol: freely soluble(lit.)
diethyl ether: freely soluble(lit.)
water: insoluble(lit.)
Densità
0.966 g/mL at 25 °C (lit.)
Stringa SMILE
CCOc1ccccc1
InChI
1S/C8H10O/c1-2-9-8-6-4-3-5-7-8/h3-7H,2H2,1H3
DLRJIFUOBPOJNS-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
Ethoxybenzene (Phenetole) was used as an analyte in assaying the performance of the porous graphitic carbon (PGC) particles.
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
H Ohi et al.
Xenobiotica; the fate of foreign compounds in biological systems, 22(11), 1329-1337 (1992-11-01)
1. The effects of oxygen concentration were studied on the metabolic pathways of anisole homologues (anisole, phenetole and isopropoxybenzene) catalysed by liver microsomes from phenobarbital-treated rats. 2. With increase of oxygen concentration, the rate of anisole o-hydroxylation reached a plateau
Wojciech Piatkowski et al.
Journal of chromatography. A, 1003(1-2), 73-89 (2003-08-06)
The competitive adsorption behavior of the binary mixture of phenetole (ethoxy-benzene) and propyl benzoate in a reversed-phase system was investigated. The adsorption equilibrium data of the single-component systems were acquired by frontal analysis. The same data for binary mixtures were
Niklas Helle et al.
European journal of mass spectrometry (Chichester, England), 25(1), 142-156 (2019-02-19)
The vibronic structure of the first electronically excited state S1 and ionic ground state D0 of phenetole has been investigated by means of resonance enhanced multi photon ionization (REMPI) and mass analyzed threshold ionization (MATI) spectroscopy. The vibronic levels were
David S Jensen et al.
Journal of chromatography. A, 1218(46), 8362-8369 (2011-10-19)
Porous graphitic carbon (PGC) particles were functionalized/passivated in situ in packed beds at elevated temperature with neat di-tert-amylperoxide (DTAP) in a column oven. The performance of these particles for high performance liquid chromatography (HPLC) was assayed before and after this
Isamu Shiina et al.
Bioorganic & medicinal chemistry, 15(24), 7599-7617 (2007-10-02)
Two new synthetic pathways to the anti-cancer agent tamoxifen and its derivatives were developed. The first route involved the aldol reaction of benzyl phenyl ketone with acetaldehyde followed by Friedel-Crafts substitution with anisole in the presence of Cl(2)Si(OTf)(2) to produce
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.