138827
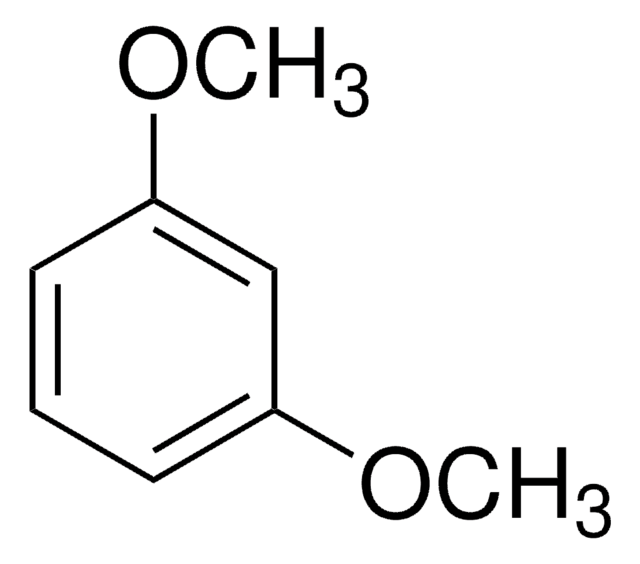
1,3,5-Trimethoxybenzene
ReagentPlus®, ≥99%
Sinonimo/i:
Phloroglucinol trimethyl ether
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H3(OCH3)3
Numero CAS:
Peso molecolare:
168.19
Beilstein:
1307993
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Nome Commerciale
ReagentPlus®
Saggio
≥99%
Stato
solid
P. ebollizione
255 °C (lit.)
Punto di fusione
50-53 °C (lit.)
Stringa SMILE
COc1cc(OC)cc(OC)c1
InChI
1S/C9H12O3/c1-10-7-4-8(11-2)6-9(5-7)12-3/h4-6H,1-3H3
LKUDPHPHKOZXCD-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
1,3,5-Trimethoxybenzene effectively cleaves p-methoxybenzyl protecting group on various alcohols and acids. It is the major scent compound present in Chinese rose species.
Applicazioni
1,3,5-Trimethoxybenzene was used to study the photodeoxygenation of 1,2-benzodiphenylene sulfoxide. It was employed as secondary standard in quantitative proton NMR spectroscopy of pharmaceuticals.
Note legali
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Xichen Cai et al.
The journal of physical chemistry. A, 111(10), 1788-1791 (2007-02-14)
One-electron oxidation of alcohols such as methanol, ethanol, and 2-propanol by 1,3,5-trimethoxybenzene radical cation (TMB*+) in the excited state (TMB*+*) was observed during the two-color two-laser flash photolysis. TMB*+ was formed by the photoinduced bimolecular electron-transfer reaction from TMB to
Tao Fang et al.
Journal of the American Chemical Society, 134(17), 7545-7552 (2012-04-06)
The development of selectively protected monosaccharide building blocks that can reliably be glycosylated with a wide variety of acceptors is expected to make oligosaccharide synthesis a more routine operation. In particular, there is an urgent need for the development of
Shuiqin Wu et al.
Plant physiology, 135(1), 95-102 (2004-05-04)
1,3,5-Trimethoxybenzene is a key component of the Chinese rose odor. This compound is synthesized in three successive methylation steps from phloroglucinol, the initial precursor. A novel, to our knowledge, phloroglucinol O-methyltransferase (POMT) characterized here methylates the first step to produce
O Chassany et al.
Alimentary pharmacology & therapeutics, 25(9), 1115-1123 (2007-04-19)
Abdominal pain is the predominant symptom in irritable bowel syndrome patients. Phloroglucinol and its methylated derivative are antispasmodic agents acting on smooth muscle. To evaluate the efficacy of phloroglucinol/trimethylphloroglucinol on pain intensity during an acute exacerbation of pain of irritable
Xichen Cai et al.
The journal of physical chemistry. A, 111(22), 4743-4747 (2007-05-08)
Bimolecular hole transfer quenching of the 1,3,5-trimethoxybenzene radical cation (TMB*+) in the excited state (TMB*+*) by hole quenchers (Q) such as biphenyl (Bp), naphthalene (Np), anisole (An), and benzene (Bz) with higher oxidation potentials than that of TMB was directly
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 138827-50G | 4061838732460 |
| 138827-10G | 4061838732453 |
| 138827-250G | 4061832879864 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.