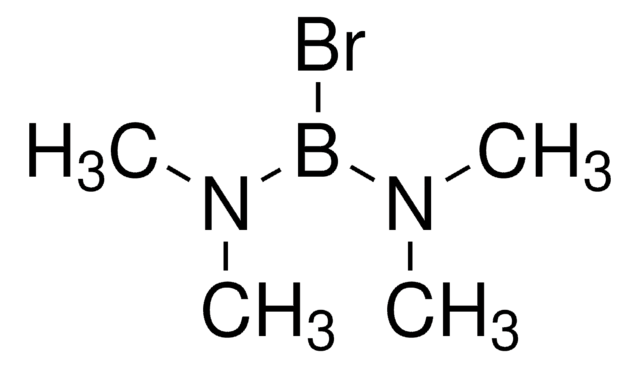
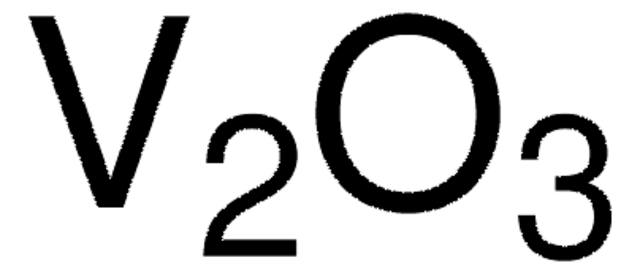230367
Boron tribromide
≥99.99%
Sinonimo/i:
Tribromoboron
About This Item
Prodotti consigliati
Densità del vapore
8.6 (vs air)
Tensione di vapore
40 mmHg ( 14 °C)
Saggio
≥99.99%
Forma fisica
liquid
P. eboll.
~90 °C (lit.)
Punto di fusione
−46 °C (lit.)
Densità
2.60 g/mL at 20 °C (lit.)
Stringa SMILE
BrB(Br)Br
InChI
1S/BBr3/c2-1(3)4
ILAHWRKJUDSMFH-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Drug intermediate 6-nitro-L-DOPA
- Luminescent polystyrene derivatives with sterically protected carbazolylborane moieties
- High-quality boron-doped graphene via Wurtz-type reductive coupling reaction
- Mercapto-(+)-methamphetamine haptens for synthesis of (+)-methamphetamine conjugate vaccines with improved epitope densities
- Micrometer-sized organic molecule-DNA hybrid structures
- Borane complexes via electrophilic aromatic borylation reactions
- A 5-HT2C receptor agonist
- Biphenyl-derivatives possessing tertiary amino groups as β-secretase(BACE1) inhibitors for the treatment of Alzheimer′s disease
- A highly near-IR region fluorescent p-extended boron aza-dipyrromethene moiety unit
- Tetrahydroisoquinoline derivatives via intramolecular cyclization of methoxy-substituted N-phenethylimides
Accessorio
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Eye Dam. 1 - Skin Corr. 1A
Codice della classe di stoccaggio
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.