151610
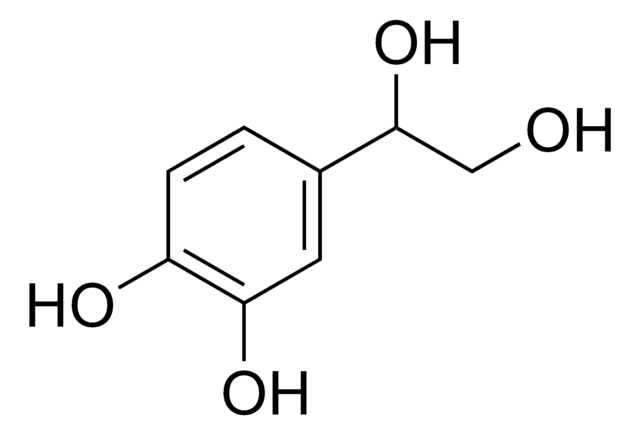
DL-3,4-Dihydroxymandelic acid
95%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(HO)2C6H3CH(OH)CO2H
Numero CAS:
Peso molecolare:
184.15
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
95%
Punto di fusione
136-137 °C (dec.) (lit.)
Gruppo funzionale
carboxylic acid
hydroxyl
Stringa SMILE
OC(C(O)=O)c1ccc(O)c(O)c1
InChI
1S/C8H8O5/c9-5-2-1-4(3-6(5)10)7(11)8(12)13/h1-3,7,9-11H,(H,12,13)
RGHMISIYKIHAJW-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Metabolite of norepinephrine.
Applicazioni
DL-3,4-Dihydroxymandelic acid was used in the simultaneous analysis of 4-hydroxy-3-methoxymandelic acid and 4-hydroxy- 3-methoxyphenylacetic acid in urine. It was also used to study the changes in body temperature.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
W X Dong et al.
Journal of the autonomic nervous system, 44(2-3), 109-117 (1993-08-01)
Pre-synaptic endings of the sympathetic nervous fibers control the metabolism of catecholamines, particularly inactivating norepinephrine after its neuronal recapture. The present study was carried out to investigate this segment of the metabolism of catecholamines through measurements of DHPG, DOMA and
S J Ley et al.
Research in veterinary science, 61(2), 172-173 (1996-09-01)
The threshold response to a mechanical nociceptive stimulus was significantly lower on the lame hind limb of lame cows than on the same limb of sound cows. There were no significant differences between the concentrations of cortisol, noradrenaline, adrenaline or
K E O'Connor et al.
Journal of bacteriology, 183(3), 928-933 (2001-02-24)
Pseudomonas putida F6 was found to metabolize p-hydroxyphenylacetic acid through 3,4-dihydroxyphenylacetic acid, 3,4-dihydroxymandelic acid, and 3,4-dihydroxybenzaldehyde. Cell extracts of P. putida F6 catalyze the NAD(P)H-independent hydroxylation of p-hydroxyphenylacetic acid to 3,4-dihydroxyphenylacetic acid which is further oxidized to 3,4-dihydroxymandelic acid. Oxidation
J M Midgley et al.
Journal of neurochemistry, 55(3), 842-848 (1990-09-01)
Acidic metabolites of a number of biogenic amines have been identified and quantified by reaction with either acetic or propionic anhydride in the aqueous phase followed by extraction into ethyl acetate, esterification of carboxyl groups with ditrifluoromethylbenzyl bromide (DTFMBzBr), and
T R Kingsley et al.
Journal of gerontology, 46(4), B135-B141 (1991-07-01)
Adrenal catecholamines (CA) were measured in 6-, 18-, and 30-mo Lobund-Wistar rats (LWR) maintained under germ-free or conventional conditions and fed either ad libitum or a restricted (70% of adult ad libitum) diet. Levels of dopamine (DA), norepinephrine (NE), epinephrine
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.