144916
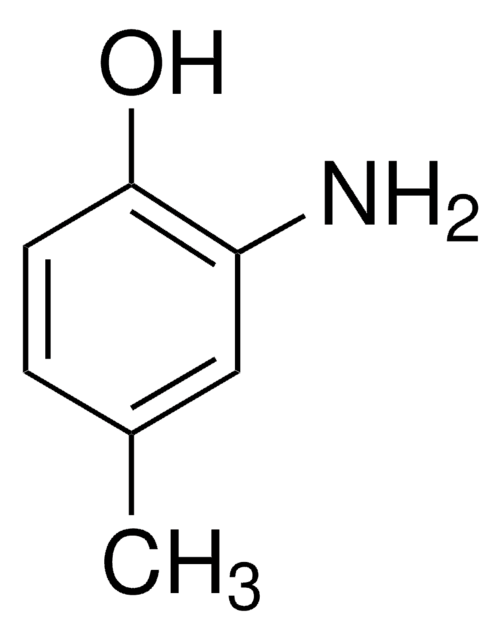
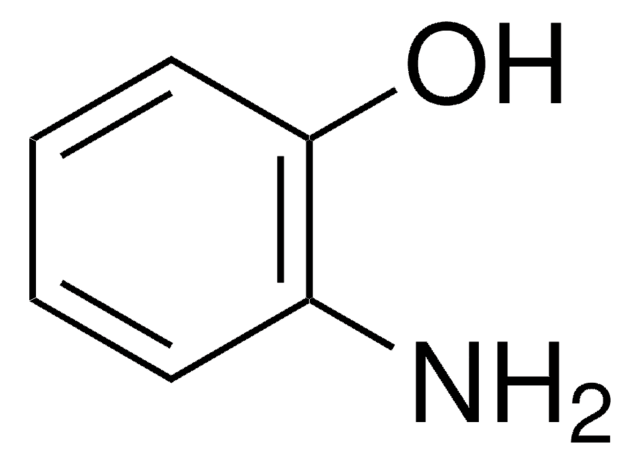
2-Amino-5-methylphenol
98%
Sinonimo/i:
2-Hydroxy-4-methylaniline, 4-Amino-3-hydroxytoluene, 6-Amino-m-cresol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
H2NC6H3(CH3)OH
Numero CAS:
Peso molecolare:
123.15
Beilstein:
386144
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Forma fisica
powder
Punto di fusione
159-162 °C (lit.)
Stringa SMILE
Cc1ccc(N)c(O)c1
InChI
1S/C7H9NO/c1-5-2-3-6(8)7(9)4-5/h2-4,9H,8H2,1H3
HCPJEHJGFKWRFM-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
2-Amino-5-methylphenol reacts with bovine hemoglobin to form 2-amino-4,4α-dihydro-4α-7-dimethyl-3H-phenoxazine-3-one, which inhibits the proliferation of Poliovirus in Vero cells. It is converted to dihydrophenoxazinone by purified human hemoglobin.
Applicazioni
2-Amino-5-methylphenol was used in the synthesis of tridentate Schiff base ligand and novel non-metallocene catalysts with phenoxy-imine ligands.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Skin Sens. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Antiviral activity of 2-amino-4,4alpha-dihydro-4alpha-7-dimethyl-3H-phenoxazine-3-one on poliovirus.
Akiko Iwata et al.
The Tohoku journal of experimental medicine, 200(3), 161-165 (2003-10-03)
2-Amino-4,4alpha-dihydro-4alpha-7-dimethyl-3H-phenoxazine-3-one (Phx), which was produced by the reaction of bovine hemoglobin with 2-amino-5-methylphenol, inhibited the proliferation of poliovirus in Vero cells between 0.25 microg/ml and 2 microg/ml with maximal antiviral acitivity at 1 microg/ml. These results suggest that Phx may
Synthesis,structural characterization and catalytic activity study of Mn(II), Fe(III), Ni(II), Cu(II) and Zn(II) complexes of quinoxaline-2-carboxalidine-2-amino-5-methylphenol: Crystal structure of the nickel (II) complex.
Sebastian M, et al.
Polyhedron, 29(15), 3014-3020 (2010)
The investigation of novel non-metallocene catalysts with phenoxy-imine ligands for ethylene (co-) polymerization.
Zhang X, et al.
Polymer International, 62(3), 419-426 (2012)
A Tomoda et al.
Bioorganic & medicinal chemistry letters, 11(8), 1057-1058 (2001-05-01)
A simple and rapid preparation method for a novel antitumor agent, 2-amino-4,4a-dihydro-4a,7-dimethyl-3H-phenoxazine-3-one (Phx) was described. The procedure included (1) the reaction of bovine hemolysates with 2-amino-5-methylphenol, (2) one-shot denaturation of hemoglobin and proteins by methanol, and removal of the denatured
A Tomoda et al.
Biochimica et biophysica acta, 1117(3), 306-314 (1992-10-27)
We found that 2-amino-5-methylphenol was converted to the dihydrophenoxazinone with a reddish brown color by purified human hemoglobin, lysates of human erythrocytes, and human erythrocytes. The reddish brown compound was identified as 2-amino-4,4 alpha-dihydro-4 alpha,7-dimethyl-3H-phenoxazin-3-one by the measurement of NMR
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.