126462
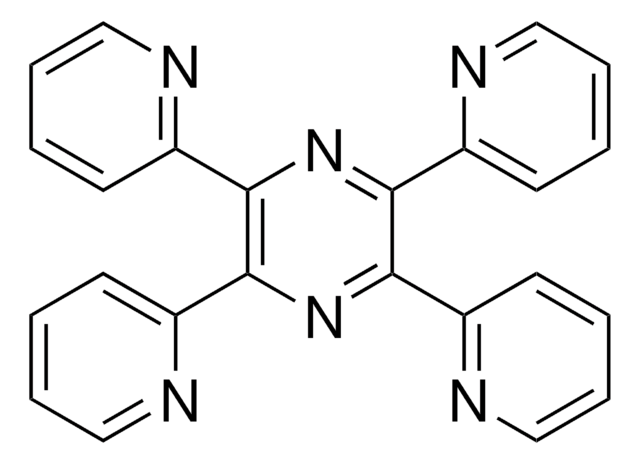
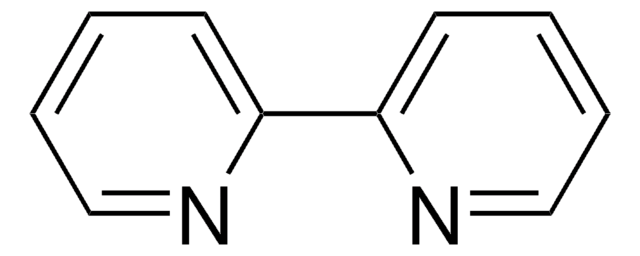
6,7-Dimethyl-2,3-di(2-pyridyl)quinoxaline
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C20H16N4
Numero CAS:
Peso molecolare:
312.37
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
98%
Stato
powder
Punto di fusione
191-193 °C (lit.)
Stringa SMILE
Cc1cc2nc(-c3ccccn3)c(nc2cc1C)-c4ccccn4
InChI
1S/C20H16N4/c1-13-11-17-18(12-14(13)2)24-20(16-8-4-6-10-22-16)19(23-17)15-7-3-5-9-21-15/h3-12H,1-2H3
NACXMBPTPBZQHY-UHFFFAOYSA-N
Categorie correlate
Applicazioni
6,7-Dimethyl-2,3-di-(2-pyridyl)quinoxaline has been used as an internal standard to investigate the clinical pharmacokinetics of nelfinavir mesylate, a potent inhibitor of HIV-1 protease.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
E Y Wu et al.
Journal of chromatography. B, Biomedical sciences and applications, 695(2), 373-380 (1997-08-01)
Nelfinavir mesylate, a potent and orally bioavailable inhibitor of HIV-1 protease (Ki=2 nM), has undergone Phase III clinical evaluation in a large population of HIV-positive patients. A high-performance liquid chromatography analytical method was developed to determine the pharmacokinetic parameters of
B Louveau et al.
Biomedical chromatography : BMC, 30(12), 2009-2015 (2016-06-10)
A precise and accurate high-performance liquid chromatography (HPLC) quantification method of rifampicin in human plasma was developed and validated using ultraviolet detection after an automatized solid-phase extraction. The method was validated with respect to selectivity, extraction recovery, linearity, intra- and
Sara Baldelli et al.
Therapeutic drug monitoring, 36(6), 739-745 (2014-04-18)
Recently, the European Medicines Agency (EMA) has released new guidelines on the validation of bioanalytical methods. In this work, we compared the analytical performance of 2 high-performance liquid chromatography with tandem mass spectrometry methods designed for the quantification of the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.