116416
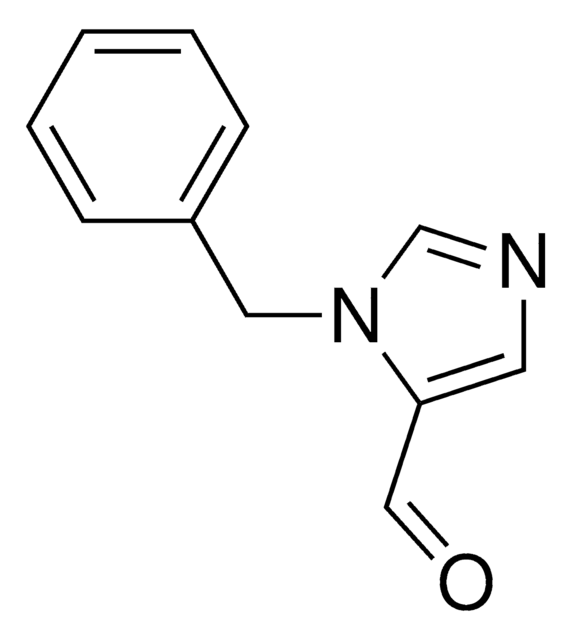
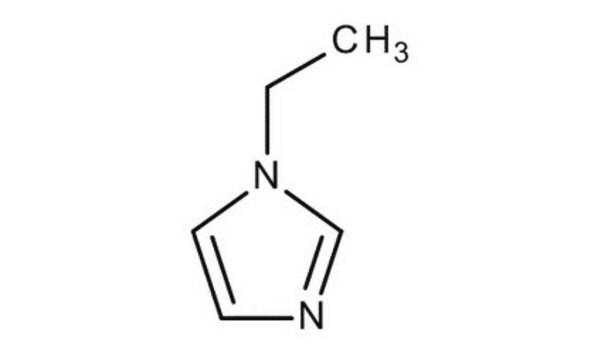
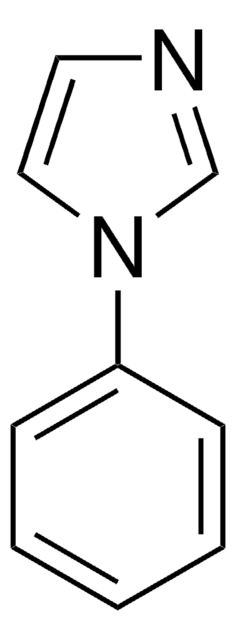
1-Benzylimidazole
99%
Sinonimo/i:
1-(Phenylmethyl)-1H-imidazole, 1-Benzyl-1H-imidazole, N-Benzylimidazole
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C10H10N2
Numero CAS:
Peso molecolare:
158.20
Beilstein:
114571
Numero CE:
Numero MDL:
Codice UNSPSC:
12352005
eCl@ss:
39161001
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
99%
P. eboll.
310 °C (lit.)
Punto di fusione
68-70 °C (lit.)
Stringa SMILE
C(c1ccccc1)n2ccnc2
InChI
1S/C10H10N2/c1-2-4-10(5-3-1)8-12-7-6-11-9-12/h1-7,9H,8H2
KKKDZZRICRFGSD-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
1-Benzylimidazole has been used to prepare cyclodextrin-ionic liquid polymer (βCD-BIMOTs-TDI).
Azioni biochim/fisiol
1-Benzylimidazole is a CYP inhibitor that inhibits the biotransformation of MeO-BDEs (methoxylated-brominated diphenyl ethers) to OH-BDEs (hydroxylated) in fishes.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Jérémie Doiron et al.
European journal of medicinal chemistry, 46(9), 4010-4024 (2011-06-28)
A series of bis- and mono-benzonitrile or phenyl analogues of letrozole 1, bearing (1,2,3 and 1,2,5)-triazole or imidazole, were synthesized and screened for their anti-aromatase activities. The unsubstituted 1,2,3-triazole 10a derivative displayed inhibitory activity comparable with that of the aromatase
José María Navas et al.
Environmental toxicology and chemistry, 22(4), 830-836 (2003-04-11)
Xenobiotics can induce cytochrome P4501A (CYP1A) by ligand binding to the aryl hydrocarbon receptor (AhR). Typical AhR ligands are polycyclic aromatic compounds with planar molecular conformation. The present work investigated the ability of the N-imidazole derivative, 1-benzylimidazole (BIM), to induce
P Rothenbach et al.
Journal of applied physiology (Bethesda, Md. : 1985), 83(2), 530-536 (1997-08-01)
This study examines the hypothesis that intestinal ischemia-reperfusion (I/R) injury contributes to renal dysfunction by altered renal eicosanoid release. Anesthetized Sprague-Dawley rats underwent 60 min of sham or superior mesenteric artery (SMA) occlusion with 60 min of reperfusion. The I/R
A Grothusen et al.
Archives of toxicology, 71(1-2), 64-71 (1996-01-01)
Liver microsomes are a frequently used probe to investigate the phase I metabolism of xenobiotics in vitro. Structures containing nucleophilic hetero-atoms are possible substrates for cytochrome P450 enzymes (P450) and flavin-containing monooxygenases (FMO). Both enzymes are located in the endoplasmatic
Fredrik Jernerén et al.
Lipids, 47(7), 707-717 (2012-05-01)
(8R)-Hydroperoxy-(9Z,12Z)-octadecadienoic acid (8-HPODE) is formed by aspergilli as an intermediate in biosynthesis of oxylipins with effects on sporulation. 8-HPODE is transformed by separate diol synthases to (5S,8R)-dihydroxy- and (8R,11S)-dihydroxy-(9Z,12Z)-octadecadienoic acids (5,8- and 8,11-DiHODE). The former is formed by the cytochrome
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![2,5-Dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c]pyrrole-1,4-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/209/681/63a4048f-a2a7-496b-814d-ccb4b5b76124/640/63a4048f-a2a7-496b-814d-ccb4b5b76124.png)