Alle Fotos(2)
Wichtige Dokumente
H43407
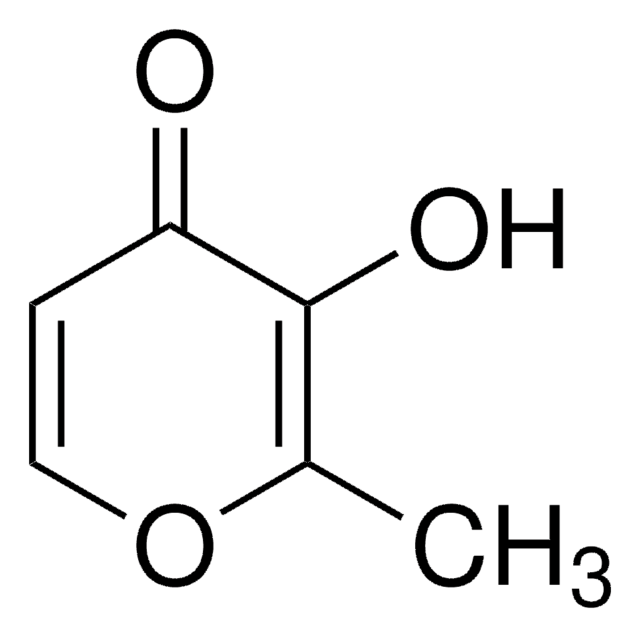
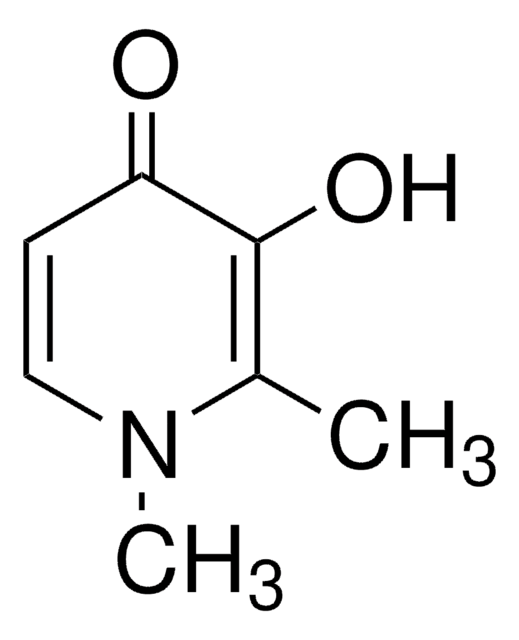
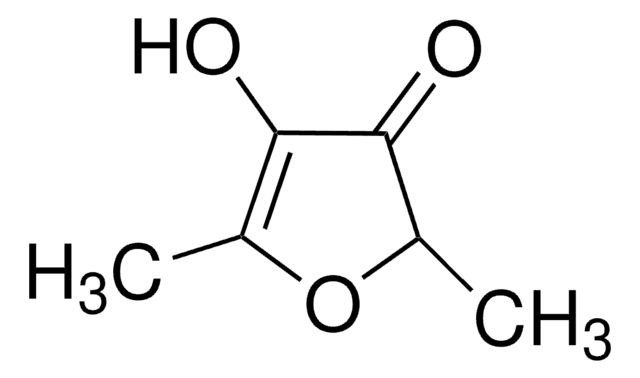
3-Hydroxy-2-methyl-4-pyron
99%
Synonym(e):
3-Hydroxy-2-methyl-4H-pyran-4-on, Maltol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C6H6O3
CAS-Nummer:
Molekulargewicht:
126.11
Beilstein:
112169
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
99%
Selbstzündungstemp.
1364 °F
Expl.-Gr.
25 %
mp (Schmelzpunkt)
160-164 °C (lit.)
SMILES String
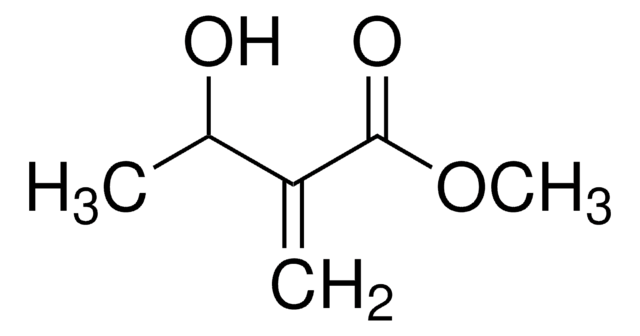
CC1=C(O)C(=O)C=CO1
InChI
1S/C6H6O3/c1-4-6(8)5(7)2-3-9-4/h2-3,8H,1H3
InChIKey
XPCTZQVDEJYUGT-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Anwar Anwar-Mohamed et al.
Toxicology in vitro : an international journal published in association with BIBRA, 21(4), 685-690 (2007-02-24)
Maltol is used extensively as a flavor-enhancing agent, food preservative, antioxidant, and also in cosmetic and pharmaceutical formulations. However, a number of studies have shown that maltol may induce carcinogenicity and toxicity but the mechanisms involved remain unknown. Therefore, we
Tamás Jakusch et al.
Dalton transactions (Cambridge, England : 2003), (13)(13), 2428-2437 (2009-03-18)
The interactions of various insulin mimetic oxovanadium(IV) compounds with serum proteins were studied in model systems and in ex vivo samples. For the modeling study, an earlier in situ method was extended and applied to the formation of ternary complexes
Sílvia Chaves et al.
Journal of inorganic biochemistry, 114, 38-46 (2012-06-13)
The O,S-donor analogues of maltol and deferiprone (DMHP), respectively, thiomaltol and DMHTP, have been investigated in solution for their iron-complexation ability, as well as their electrochemical behaviors, in the presence and absence of iron, aimed at the rationalization of their
Christoph J Jocher et al.
Inorganic chemistry, 47(18), 7951-7953 (2008-08-19)
The synthesis, characterization, and photophysical properties are reported for several Ln(III) complexes of a tetradentate chelate, 5LIO-MAM, derived from the common flavor enhancer "maltol". Eu(III), Yb(III), and Nd(III) form stable ML2 complexes in aqueous solution that emit in the red
Stefano Amatori et al.
The Journal of organic chemistry, 77(5), 2207-2218 (2012-02-03)
The N,N'-bis[(3-hydroxy-4-pyron-2-yl)methyl]-N,N'-dimethylethylendiamine (malten) and 4,10-bis[(3-hydroxy-4-pyron-2-yl)methyl]-1,7-dimethyl-1,4,7,10-tetraazacyclododecane (maltonis) were synthesized and characterized. The acid-base behavior, structural characterizations, and biochemical studies in aqueous solution were reported. Each compound contains two 3-hydroxy-2-methyl-4-pyrone units (maltol) symmetrically spaced by a polyamine fragment, the 1,4-dimethylethylendiamine (malten), or
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.