Alle Fotos(2)
Wichtige Dokumente
412929
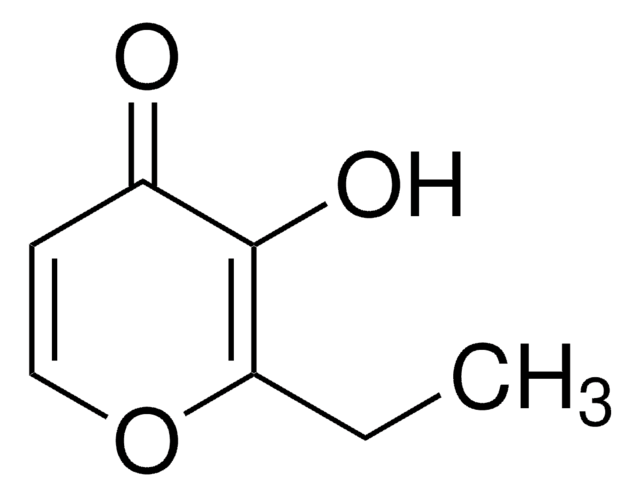
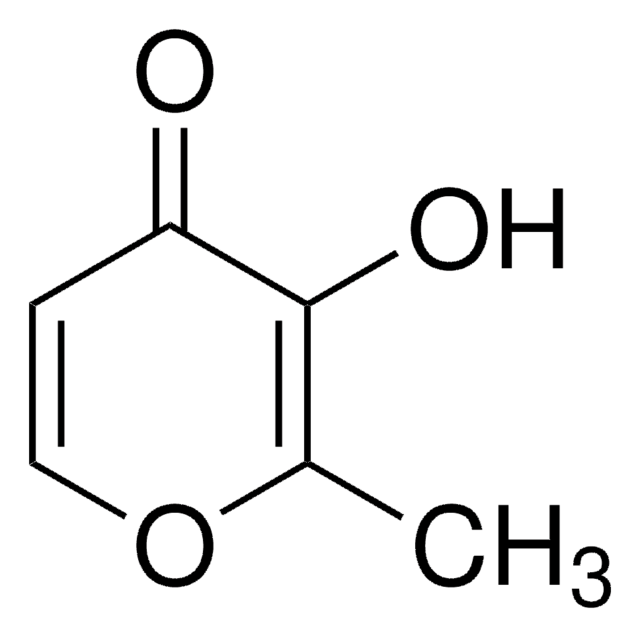
2-Ethyl-3-hydroxy-4H-pyran-4-on
99%
Synonym(e):
Ethylmaltol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C7H8O3
CAS-Nummer:
Molekulargewicht:
140.14
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
99%
Form
solid
mp (Schmelzpunkt)
85-95 °C (lit.)
Funktionelle Gruppe
ether
ketone
SMILES String
CCC1=C(O)C(=O)C=CO1
InChI
1S/C7H8O3/c1-2-6-7(9)5(8)3-4-10-6/h3-4,9H,2H2,1H3
InChIKey
YIKYNHJUKRTCJL-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- Synthesis and Anti-Oomycete Mechanism: Research focused on the synthesis of sulfonate derivatives of Ethyl Maltol, incorporating 2-Ethyl-3-hydroxy-4H-pyran-4-one, and their application in combating oomycete pathogens, providing insights into the preliminary mechanisms that enhance agricultural productivity and disease management (Xing et al., 2022).
- Redox Activity in Co(III) Complexes: A study examined the effects of replacing traditional tripodal donors with two 2N chelators in Co(III) complexes containing maltolato (a derivative of 2-Ethyl-3-hydroxy-4H-pyran-4-one) on their redox behavior and cytotoxic activity, which is crucial for the development of novel therapeutic agents (Nagy et al., 2021).
- Characterization of Wine Aroma Compounds: The role of 2-Ethyl-3-hydroxy-4H-pyran-4-one in the characterization of odor-active compounds in various cherry wines was explored using gas chromatography-mass spectrometry and olfactometry, highlighting its significance in enhancing sensory attributes and wine quality (Niu et al., 2011).
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Volatile and semi-volatile components of oak wood chips analysed by accelerated solvent extraction (ASE) coupled to gas chromatography-mass spectrometry (GC-MS).
Vichi S, et al.
Food Chemistry, 102(4), 1260-1269 (2007)
Carlyle Ribeiro Lima et al.
Molecules (Basel, Switzerland), 19(7), 9591-9605 (2014-07-09)
Tyrosinase is a key enzyme in melanin synthesis and widely distributed in plants and animals tissues. In mammals, this enzyme is related to pigment production, involved in wound healing, primary immune response and it can also contribute to catecholamines synthesis
Mutlu Dilsiz Aytemir et al.
Archiv der Pharmazie, 337(5), 281-288 (2004-04-20)
In this study, thirteen 3-hydroxy-6-methyl-2-substituted 4H-pyran-4-one derivatives were synthesized for the evaluation of their potential anticonvulsant activity. Mannich bases were prepared by the reaction of substituted piperazine derivatives with allomaltol and formaline. The structures of the synthesized compounds were confirmed
Yunwei Niu et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 879(23), 2287-2293 (2011-07-06)
To characterize the aroma of cherry wine, five samples were analyzed by quantitative descriptive sensory analysis, gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O). The aroma of cherry wines was described by 6 sensory terms as fruity, sour, woody, fermentation
R D Abeysinghe et al.
European journal of nuclear medicine, 21(10), 1141-1147 (1994-10-01)
In order to identify new compounds which label platelets without affecting their function, three classes of metal chelating agents have been compared with oxine for their efficiency of indium-113m platelet labelling and for their short- and long-term effects on platelet
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.