1342059
USP
Insulin glargine
United States Pharmacopeia (USP) Reference Standard
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Produtos recomendados
grau
pharmaceutical primary standard
família API
insulin
fabricante/nome comercial
USP
aplicação(ões)
pharmaceutical (small molecule)
Formato
neat
temperatura de armazenamento
−20°C
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including MSDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
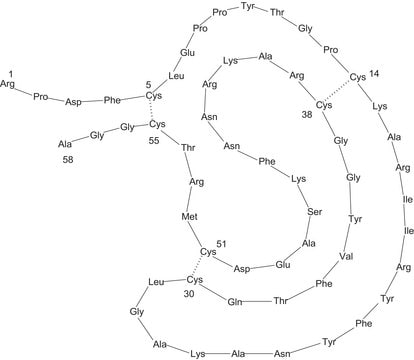
Insulin Glargine, a peptide produced by recombinant DNA techniques is composed of two chains - the A-chain (21 amino acids) and the B-chain (32 amino acids), making up a total of 53 amino acids. Its primary structure is almost identical to that of Human Insulin, except for the A21 position, where it has Gly instead of Asn. It has two additional amino acids, Arg (B31) and Arg (B32), located at the end of the B-chain.
For further information and support please go to the website of the issuing Pharmacopoeia.
Insulin Glargine, a peptide produced by recombinant DNA techniques is composed of two chains - the A-chain (21 amino acids) and the B-chain (32 amino acids), making up a total of 53 amino acids. Its primary structure is almost identical to that of Human Insulin, except for the A21 position, where it has Gly instead of Asn. It has two additional amino acids, Arg (B31) and Arg (B32), located at the end of the B-chain.
Aplicação
Insulin glargine USP reference standard suitable for use in specified USP compendial tests and assays.
Also used to prepare standard, standard stock and system suitability solutions for assay by liquid chromatography in conjunction with a UV detector according to the given below monographs of United States Pharmacopeia (USP):
Also used to prepare standard, standard stock and system suitability solutions for assay by liquid chromatography in conjunction with a UV detector according to the given below monographs of United States Pharmacopeia (USP):
- Insulin Glargine Injection
- Insulin Glargine
- 〈121〉 Insulin Assays
Nota de análise
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
Outras notas
This product is part of the USP Biologics program.
Sales restrictions may apply.
produto relacionado
Nº do produto
Descrição
Preços
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
nwg
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica