1242000
USP
Erythromycin
United States Pharmacopeia (USP) Reference Standard
About This Item
Produtos recomendados
grau
pharmaceutical primary standard
família API
erythromycin
fabricante/nome comercial
USP
aplicação(ões)
pharmaceutical (small molecule)
Formato
neat
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
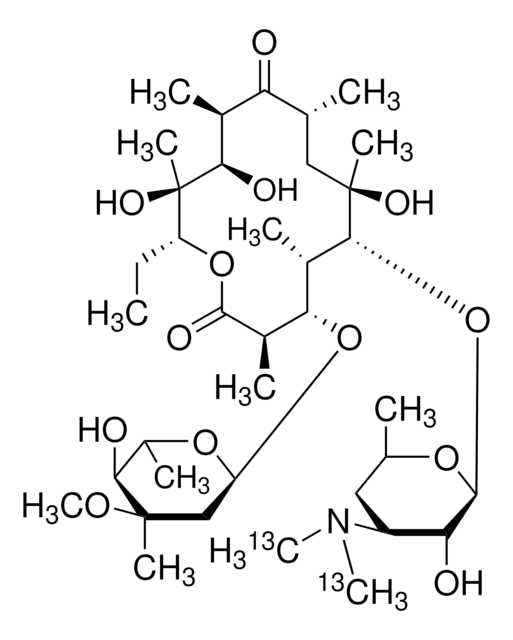
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O
InChI
1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
chave InChI
ULGZDMOVFRHVEP-RWJQBGPGSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Erythromycin USP reference standard is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including MSDS and any product information leaflets, have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Aplicação
- Erythromycin Stearate
- Erythromycin Ethylsuccinate
- Erythromycin Lactobionate for Injection
- Erythromycin Ointment
- Erythromycin Pledgets
- Erythromycin Injection
Ações bioquímicas/fisiológicas
Antimicrobial Spectrum: This product acts against both gram-negative and gram-positive bacteria.
Atenção
Nota de preparo
Outras notas
Informações legais
produto relacionado
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lamentamos, não temos COA para este produto disponíveis online no momento.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica