L5135
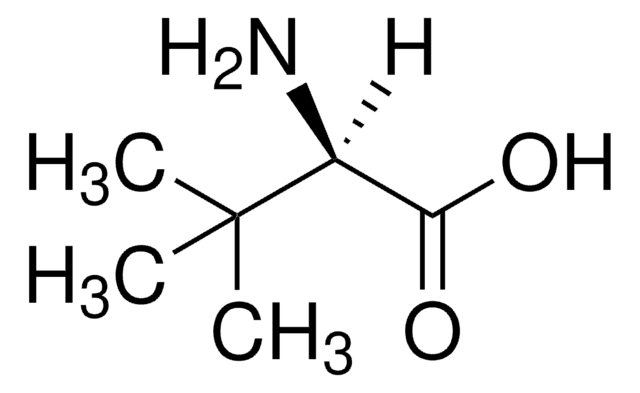
L-Leucine Dehydrogenase from Bacillus cereus
lyophilized powder, ≥60 units/mg protein
Sinônimo(s):
L-Leucine:NAD+ oxidoreductase (deaminating)
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Número CAS:
Número MDL:
Código UNSPSC:
12352204
NACRES:
NA.54
Produtos recomendados
Formulário
lyophilized powder
Nível de qualidade
atividade específica
≥60 units/mg protein
peso molecular
245 kDa
temperatura de armazenamento
−20°C
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
L-Leucine Dehydrogenase is a member of the amino acid dehydrogenase family.
Aplicação
L-Leucine Dehydrogenase from Bacillus cereus has been used to determine the branched-chain amino acids (BCAA) spectrophotometrically in serum samples.
Ações bioquímicas/fisiológicas
Leucine Dehydrogenase is a nicotinamide adenine dinucleotide hydrogen (NADH)-dependent oxidoreductase. It is involved in catalyzing the reductive amination of aliphatic 2-oxo-acids to their respective L-amino acids.
Definição da unidade
One unit will convert 1.0 μmole of L‑leucine to α-ketoisocaproate per min at pH 10.5 at 37 °C.
Outras notas
contains lysine
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
K Selber et al.
Journal of chromatography. B, Biomedical sciences and applications, 743(1-2), 21-30 (2000-08-15)
Mathematical strategies were applied to optimise the extraction of recombinant leucine dehydrogenase from E. coli homogenates and endoglucanase 1 from culture filtrates of Trichoderma reesei in polyethylene glycol-phosphate systems. The goal was to test mathematical tools which could facilitate the
M B Ansorge et al.
Applied microbiology and biotechnology, 53(6), 668-673 (2000-08-05)
The established Escherichia coli expression vectors ptrc99a, pKK223-3, pPLlambda, pAsk75, pRA95, and pRA96, which differ in copy number, mode of induction, selection marker, and use of par sequences for stabilization, were investigated for the stable expression of recombinant L-leucine dehydrogenase
S Guangdong et al.
The Journal of antibiotics, 54(1), 66-73 (2001-03-28)
Shengjimycin is a group of 4"-acylated spiramycins with 4"-isovalerylspiramycin as the major component, produced by recombinant S. spiramyceticus F21 harboring a 4"-O-acyltransferase gene from S. mycarofaciens 1748. A stable bioengineered strain of Streptomyces spiramyceticus WSJ-1 was constructed by integrating the
Peng-Hu Zhang et al.
Sheng wu gong cheng xue bao = Chinese journal of biotechnology, 23(2), 268-272 (2007-04-28)
The purification and the characteristics of an enzyme from Morganella morganii J-8, which could produce d-pseudoephedrine from 1-phenyl-2-methylamine-acetone, were performed in this study. In this research, first, cells were disrupted by ultrasonic treatment at 4 degrees C. The carbonyl enantioselective
Tatyana A Muranova et al.
Acta crystallographica. Section D, Biological crystallography, 58(Pt 6 Pt 2), 1059-1062 (2002-05-31)
Leucine dehydrogenase is an octameric enzyme which belongs to the superfamily of amino-acid dehydrogenases and catalyses the reversible oxidative deamination of leucine to 2-ketoisocaproate, with the corresponding reduction of the cofactor NAD(+). Catalysis by this enzyme is thought to involve
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica