K3125
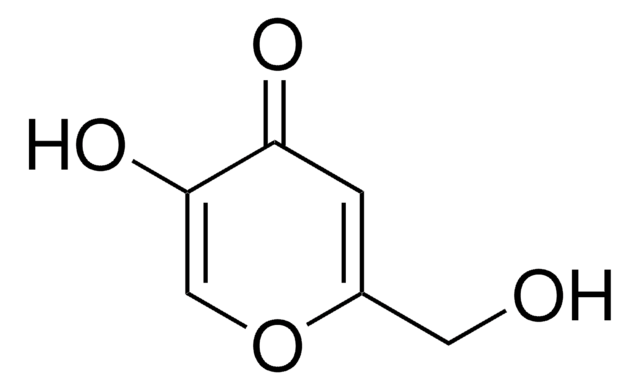
Kojic acid
≥98.5% (HPLC), powder, tyrosinase inhibitor
Sinônimo(s):
2-Hydroxymethyl-5-hydroxy-γ-pyrone, 5-Hydroxy-2-hydroxymethyl-4H-4-pyranone
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C6H6O4
Número CAS:
Peso molecular:
142.11
Beilstein:
120895
Número CE:
Número MDL:
Código UNSPSC:
12352106
ID de substância PubChem:
NACRES:
NA.77
Produtos recomendados
Nome do produto
Kojic acid,
Ensaio
≥98.5% (HPLC)
Nível de qualidade
Formulário
powder
pf
152-155 °C (lit.)
cadeia de caracteres SMILES
OCC1=CC(=O)C(O)=CO1
InChI
1S/C6H6O4/c7-2-4-1-5(8)6(9)3-10-4/h1,3,7,9H,2H2
chave InChI
BEJNERDRQOWKJM-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Kojic acid has been used:
- as an inhibitor of tyrosinase in guinea pigs pigmented hyperopic (PH)
- as a reference inhibitor standard for screening tyrosinase inhibition
- as a positive control for inhibition of tyrosinase in B16F10 melanoma cells
Ações bioquímicas/fisiológicas
Kojic acid is derived from some fungal species such as, Aspergillus, Acetobacter and Penicillium.. It halts melanin synthesis by inhibiting tyrosinase enzyme. It is used in the preparation of skin whitening cosmetics. However, kojic acid usage is minimal in cosmetics, as it induces skin irritation by its unstability and cytotoxic nature during long storage. It is an antioxidant and elicits radioprotective effects on chelating with manganese and zinc.
Tyrosinase inhibitor.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Effects of the Tyrosinase-Dependent Dopaminergic System on Refractive Error Development in Guinea Pigs
Jiang L, et al.
Investigative Ophthalmology & Visual Science, 59(11), 4631-4638 (2018)
Ka-Heng Lee et al.
European journal of medicinal chemistry, 44(8), 3195-3200 (2009-04-11)
A series of 46 curcumin related diarylpentanoid analogues were synthesized and evaluated for their anti-inflammatory, antioxidant and anti-tyrosinase activities. Among these compounds 2, 13 and 33 exhibited potent NO inhibitory effect on IFN-gamma/LPS-activated RAW 264.7 cells as compared to L-NAME
Kojic acid and its manganese and zinc complexes as potential radioprotective agents
Emami S, et al.
Bioorganic & Medicinal Chemistry Letters, 17(1), 45-48 (2007)
Xiao Hu et al.
Journal of natural products, 75(1), 82-87 (2011-12-15)
Two novel 2-arylbenzofuran dimers, morusyunnansins A and B (1 and 2), two new biflavonoids, morusyunnansins C and D (3 and 4), two new flavans, morusyunnansins E and F (5 and 6), and four known flavans (7-10) were isolated from the
Wei Yi et al.
European journal of medicinal chemistry, 46(9), 4330-4335 (2011-07-23)
Melanin play a major role in human skin protection and their biosynthesis is vital. Due to their color, they contribute to the skin pigmentation. Tyrosinase is a key enzyme involved in the first stage of melanin biosynthesis, it catalyzes the
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica