H7425
Anti-FLAG®-Peroxidase antibody produced in rabbit
IgG fraction of antiserum
Sinônimo(s):
Anti-FLAG-HRP Polyclonal Conjugate
About This Item
Produtos recomendados
Nível de qualidade
descrição
buffered aqueous solution
Formulário
lyophilized solid
técnica(s)
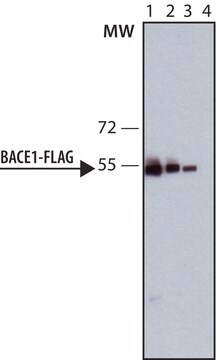
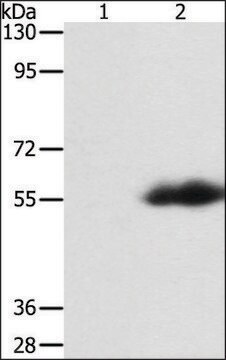
western blot: 1:2000-1:4000 using for detection of amino-terminal FLAG-BAP fusion protein in an E. coli crude cell lysate
Condições de expedição
dry ice
temperatura de armazenamento
−20°C
Descrição geral
ANTI-FLAG recognizes the FLAG epitope located on FLAG fusion proteins. The antibody reacts with N-terminal, N-terminal-Met, and C-terminal FLAG fusion proteins by immunoblotting. Specific staining is inhibited by the FLAG peptide (N-Asp-Tyr-Lys-Asp-AspAsp-Asp-Lys-C). Applications for the conjugate include Western blots and ELISA.
Epitope tags provide a method to localize gene products in a variety of cell types, study the topology of proteins and protein complexes, identify associated proteins, and characterize newly identified, low abundance, or poorly immunogenic proteins when protein specific antibodies are not available. Tagging with the FLAG peptide sequence may be done at the N-terminus, N-terminus preceded by a methionine residue, C-terminus, or at internal positions of the target protein. FLAG may also be placed in association with other tags. The small size of the FLAG tag or sequence and its high hydrophilicity tend to decrease the possibility of interference with the protein expression, proteolytic maturation, antigenicity, and function. The N-terminal FLAG peptide sequence contains a unique enterokinase cleavage site allowing it to be completely removed from the purified fusion proteins. Cleavage catalyzed by Cu2+ ions of the C-terminal FLAG peptide from a fusion protein has been reported. A sequence motif with five out of eight amino acid residues identical to the FLAG peptide is found in both rat and mouse Mg2+ dependent protein β-phosphatase, as well as in the human and bovine enzyme.
Imunogênio
Aplicação
Browse additional application references in our FLAG® Literature FLAG® Literature portal.
forma física
Informações legais
Exoneração de responsabilidade
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Chronic 3 - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica