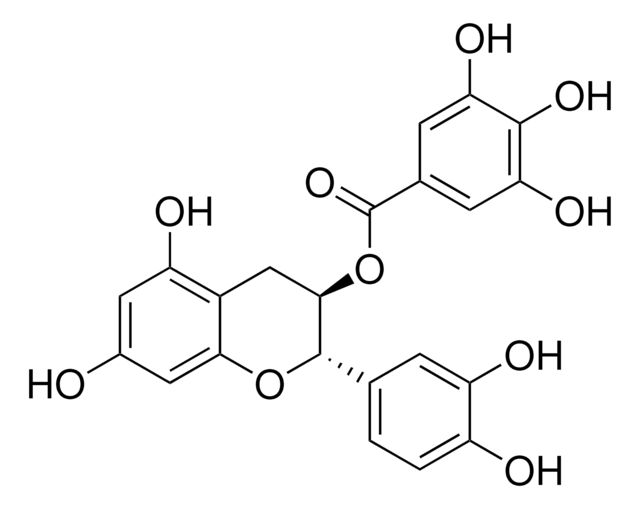
E4143
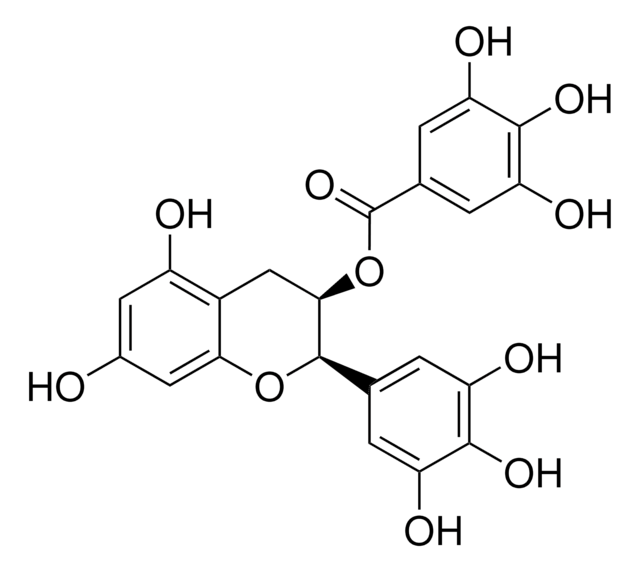
(−)-Epigallocatechin gallate
≥95%
Sinônimo(s):
(−)-cis-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol 3-gallate, (−)-cis-3,3′,4′,5,5′,7-Hexahydroxy-flavane-3-gallate, EGCG
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥95%
solubilidade
H2O: ≥5 mg/mL, clear
aplicação(ões)
metabolomics
vitamins, nutraceuticals, and natural products
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c4cc(O)c(O)c(O)c4
InChI
1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1
chave InChI
WMBWREPUVVBILR-WIYYLYMNSA-N
Informações sobre genes
human ... CYP1A2(1544)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- as an anti-tumor agent on murine TRAMP metastatic prostate cell line by cell proliferation assay, apoptosis detection and modified Boyden-chamber assay
- induces cell death in acute myeloid leukaemia through death associated protein kinase-2 pathway analyzed through cell viability, cell cycle and apoptosis assay
- as an inhibitor of osteoclast differentiation in murine preosteoclast cell line RAW264.7
- to promote myogenic differentiation
Ações bioquímicas/fisiológicas
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Sens. 1
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica