82415
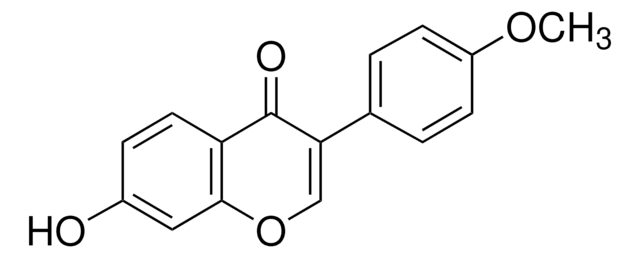
Prunetin
≥98.0% (TLC)
Sinônimo(s):
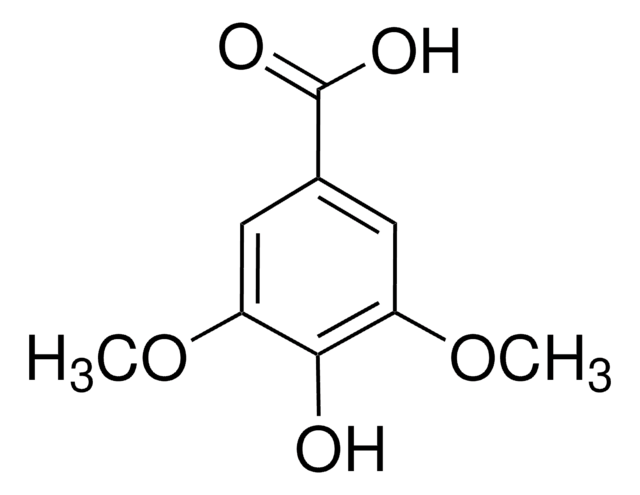
4′,5-Dihydroxy-7-methoxyisoflavone
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C16H12O5
Número CAS:
Peso molecular:
284.26
Beilstein:
292155
Número CE:
Número MDL:
Código UNSPSC:
51111800
ID de substância PubChem:
NACRES:
NA.77
Produtos recomendados
Ensaio
≥98.0% (TLC)
Formulário
powder
cadeia de caracteres SMILES
COc1cc(O)c2C(=O)C(=COc2c1)c3ccc(O)cc3
InChI
1S/C16H12O5/c1-20-11-6-13(18)15-14(7-11)21-8-12(16(15)19)9-2-4-10(17)5-3-9/h2-8,17-18H,1H3
chave InChI
KQMVAGISDHMXJJ-UHFFFAOYSA-N
Ações bioquímicas/fisiológicas
Inhibitor of both cytoplasmic and mitochondrial human liver aldehyde dehydrogenases, possibly by binding at an allosteric site.
Embalagem
Bottomless glass bottle. Contents are inside inserted fused cone.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Soon-Sen Leow et al.
Experimental gerontology, 106, 198-221 (2018-03-20)
Palm fruit juice (PFJ) containing oil palm phenolics is obtained as a by-product from oil palm (Elaeis guineensis) fruit milling. It contains shikimic acid, soluble fibre and various phenolic acids including p-hydroxybenzoic acid and three caffeoylshikimic acid isomers. PFJ has
M L Shen et al.
Journal of the American Society for Mass Spectrometry, 12(1), 97-104 (2001-01-06)
Aldehyde dehydrogenases (ALDH) are a family of enzymes primarily involved in the oxidation of various aldehydes. Most ALDH enzymes derived from mammalian sources have been shown to exist as homotetramers, consisting of four identical subunits of approximately 54 kDa. The
N S Nagarajan et al.
Natural product research, 20(2), 195-200 (2005-12-02)
Two isoflavonoids isolated from Dalbergia sympathetica were identified as 5,4'-dihydroxy-7-methoxyisoflavone (1) (Prunetin) and Prunetin-4'-O-beta-D-gentiobioside (2) (Dalsympathetin). The natural occurrence of Dalsympathetin is reported for the first time. The position of glycosylation in Dalsympathetin at 4'-position has been confirmed by 2D-NMR
Stephen W J Wang et al.
The Journal of pharmacology and experimental therapeutics, 329(3), 1023-1031 (2009-03-07)
Flavonoids have poor bioavailabilities largely because of metabolism via UDP-glucuronosyltransferases (UGTs). This study aims to further understand the functions of UGT in metabolizing genistein and apigenin, two compounds metabolized more extensively in the gut than in the liver. Because Gunn
Hyun Jae Lee et al.
Phytotherapy research : PTR, 25(8), 1196-1200 (2011-02-10)
This study investigated whether prunetin significantly affects the secretion, production and gene expression of mucin from cultured airway epithelial cells. Confluent primary rat tracheal surface epithelial (RTSE) cells were pretreated with adenosine triphosphate (ATP) for 5 min and then chased for
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica