76157
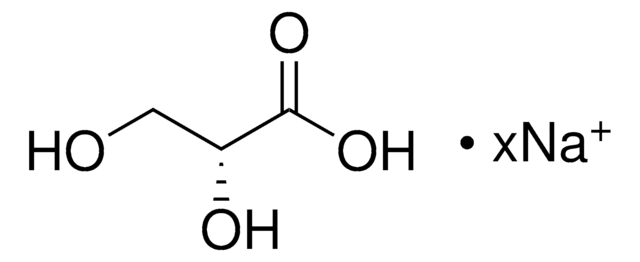
(4R)-4-Hydroxy-L-glutamic acid
≥98.0% (TLC)
Sinônimo(s):
erythro-(4R)-4-Hydroxy-L-glutamic acid, H-(2S,4R)-γ-Hydroxy-Glu-OH
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C5H9NO5
Número CAS:
Peso molecular:
163.13
Beilstein:
1725871
Número MDL:
Código UNSPSC:
12352202
ID de substância PubChem:
NACRES:
NA.28
Produtos recomendados
Nome do produto
(4R)-4-Hydroxy-L-glutamic acid, ≥98.0% (TLC)
Ensaio
≥98.0% (TLC)
Formulário
powder
atividade óptica
[α]/D 20.5±1.5°, c = 1 in H2O
cor
white
pf
171 °C
temperatura de armazenamento
−20°C
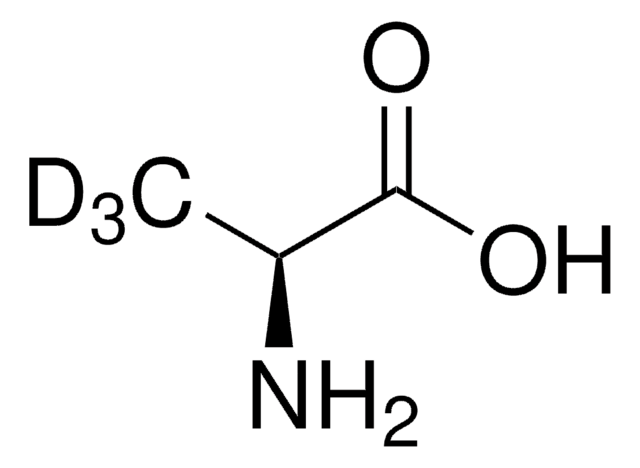
cadeia de caracteres SMILES
N[C@@H](C[C@@H](O)C(O)=O)C(O)=O
InChI
1S/C5H9NO5/c6-2(4(8)9)1-3(7)5(10)11/h2-3,7H,1,6H2,(H,8,9)(H,10,11)/t2-,3+/m0/s1
chave InChI
HBDWQSHEVMSFGY-STHAYSLISA-N
Ações bioquímicas/fisiológicas
(4R)-4-Hydroxy-L-glutamic acid or (2S,4R)-4-hydroxyglutamate was shown to activate the metabotropic glutamate receptors, mGlu1a, mGlu2, and mGlu8a in a dose-dependent manner.
Substrate for aminotransferase; pharmacological characterization at human glutamate transporter subtypes 1-3; for studying structure-activity relationships (SAR) for ionotropic and metabotropic glutamate receptors.
Embalagem
Bottomless glass bottle. Contents are inside inserted fused cone.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Metabolism of gamma-hydroxyglutamic acid. I. Conversion to alpha-hydroxy-gamma-ketoglutarate by purified glutamic-aspartic transaminase to rat liver.
A GOLDSTONE et al.
The Journal of biological chemistry, 237, 3476-3485 (1962-11-01)
A S Bessis et al.
Bioorganic & medicinal chemistry letters, 11(12), 1569-1572 (2001-06-20)
The (2S,4R)- and (2S,4S)-4-hydroxyglutamates activate cloned mGlu(1a), mGlu(2), and mGlu(8a) receptors with different potencies. Best results were obtained with the (2S,4S) isomer being almost as potent as glutamate on mGlu(1a)R and mGlu(8a)R. Data are interpreted on the basis of the
Lennart Bunch et al.
ChemMedChem, 4(11), 1925-1929 (2009-09-05)
Subtype-selective ligands are of great interest to the scientific community, as they provide a tool for investigating the function of one receptor or transporter subtype when functioning in its native environment. Several 4-substituted (S)-glutamate (Glu) analogues were synthesized, and altogether
Sebastien Alaux et al.
Journal of medicinal chemistry, 48(25), 7980-7992 (2005-12-13)
A series of nine L-2,4-syn-4-alkylglutamic acid analogues (1a-i) were synthesized in high yield and high enantiomeric excess (>99% ee) from their corresponding 4-substituted ketoglutaric acids (2a-i), using the enzyme aspartate aminotransferase (AAT) from pig heart or E. coli. The synthesized
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica