17793
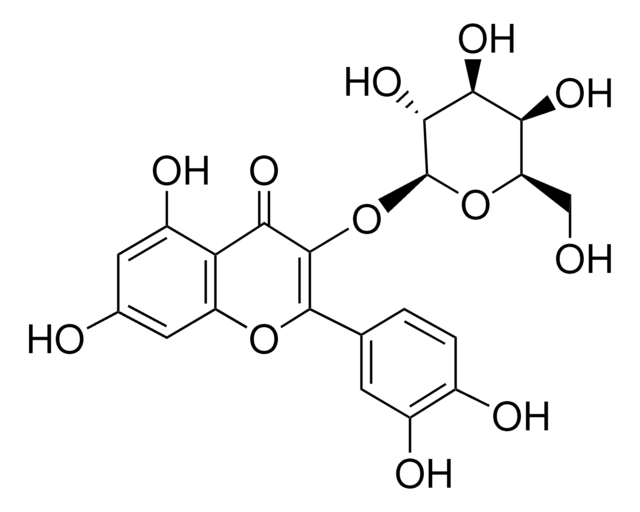
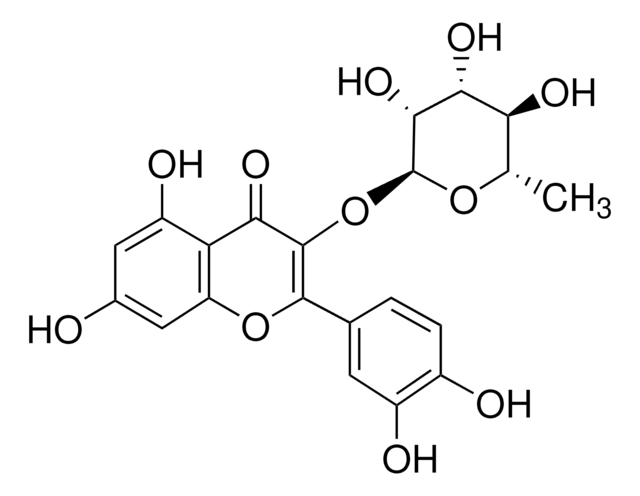
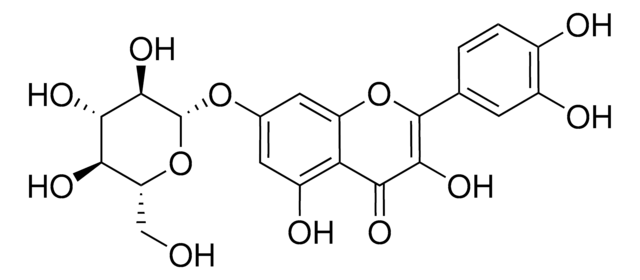
Quercetin 3-β-D-glucoside
≥90% (HPLC)
Sinônimo(s):
3,3′,4′,5,7-Pentahydroxyflavone 3-β-glucoside, Isoquercitrin
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥90% (HPLC)
aplicação(ões)
metabolomics
vitamins, nutraceuticals, and natural products
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
OC[C@H]1O[C@@H](OC2=C(Oc3cc(O)cc(O)c3C2=O)c4ccc(O)c(O)c4)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-27,29-30H,6H2/t13-,15-,17+,18-,21+/m1/s1
chave InChI
OVSQVDMCBVZWGM-QSOFNFLRSA-N
Informações sobre genes
mouse ... Hexa(15211)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- as a dietary flavonoid supplement to check its binding capacity with human small ubiquitin-related modifier 1 (SUMO1) protein using surface plasmon resonance (SPR)
- as an inhibitor for Escherichia coli adenosine triphosphate (ATP) synthase
- as an anti-aggregation agent to test its activity against β-amyloid, green fluorescent protein (GFP), and chymotrypsinogen proteins
Ações bioquímicas/fisiológicas
Embalagem
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Protocolos
HPLC Analysis of Polyphenols in Nero d'Avola Red Wine on Discovery® HS C18 (UV 280 nm)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica