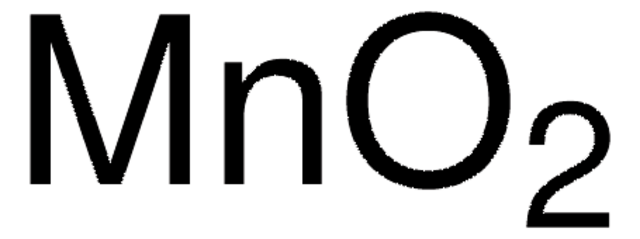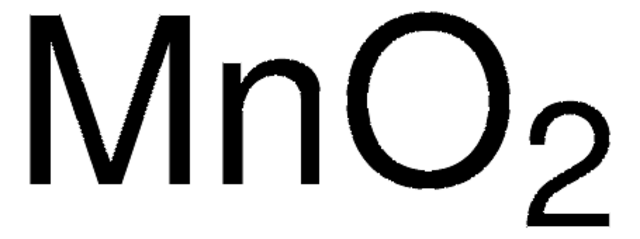310700
Manganese(IV) oxide
10 μm, reagent grade, ≥90%
Sinônimo(s):
Manganese dioxide
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
MnO2
Número CAS:
Peso molecular:
86.94
Número CE:
Número MDL:
Código UNSPSC:
12352303
ID de substância PubChem:
NACRES:
NA.55
Produtos recomendados
grau
reagent grade
Nível de qualidade
Ensaio
≥90%
Formulário
powder
tamanho de partícula
10 μm
pf
535 °C (dec.) (lit.)
cadeia de caracteres SMILES
O=[Mn]=O
InChI
1S/Mn.2O
chave InChI
NUJOXMJBOLGQSY-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Manganese(IV) oxide is an oxidizing reagent that can be used for the oxidation of propargylic alcohols, benzylic or heterocyclic alcohols, saturated alcohols, 1,2-diols, allylic alcohols to α, β-ethylenic aldehydes or ketones, and amines to aldehydes, imines, amides, and diazo compounds. It can also be used for the conversion of allylic alcohols to α, β-ethylenic esters or amides, hydration of nitriles to amides, dehydrogenation and aromatization reactions.
Aplicação
- High-oxidation-state 3d metal complexes: Explores the catalytic properties of manganese(IV) oxide within high-oxidation-state complexes for advanced organic synthesis, demonstrating its critical role in accelerating chemical reactions and enhancing yield efficiencies, beneficial for pharmaceutical and chemical industries (Cheng J et al., 2018).
- Synthesis and properties of manganese complexes: Details the synthesis of new manganese complexes that demonstrate unique redox properties, useful for understanding electron transfer processes in various chemical and environmental contexts (Baffert C et al., 2002).
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - STOT RE 2 Inhalation
Órgãos-alvo
Brain
Código de classe de armazenamento
13 - Non Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
does not flash
Ponto de fulgor (°C)
does not flash
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Manganese dioxide
Cahiez G, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Wen-Hui Kuan et al.
Journal of hazardous materials, 239-240, 152-159 (2012-09-25)
This study examined the reaction of methylene blue (MB) with tunneled manganese oxide pyrolusite regarding pH and reaction time. MB was cleaved through N-demethylation, in which reaction azure B (AB), azure A (AA), azure C (AC), and thionin (TH) were
Heng Lai et al.
ACS applied materials & interfaces, 4(5), 2325-2328 (2012-05-02)
MnO(2) nanoflakes coated on carbon nanohorns (CNHs) has been synthesized via a facile solution method and evaluated as anode for lithium-ion batteries. By using CNHs as buffer carrier, MnO(2)/CNH composite displays an excellent capacity of 565 mA h/g measured at
Xihong Lu et al.
Advanced materials (Deerfield Beach, Fla.), 24(7), 938-944 (2012-03-10)
WO3–x@Au@MnO2 core–shell nanowires (NWs) are synthesized on a flexible carbon fabric and show outstanding electrochemical performance in supercapacitors such as high specific capacitance, good cyclic stability, high energy density, and high power density. These results suggest that the WO3–x@Au@MnO2 NWs
Y Wang et al.
Journal of colloid and interface science, 380(1), 8-15 (2012-06-02)
Bio-inspired chemical approach has been developed for the surface modification and electrophoretic deposition of manganese dioxide and zirconia nanoparticles, prepared by chemical precipitation methods. Caffeic acid, trans-cinnamic acid, p-coumaric acid, and 2,4-dihydroxycinnamic acid were investigated for the surface modification of
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica